Question: 1.10 (a) For the reaction: CO + }O, C, What is the enthalpy of reaction (AH) at 298 K? (b) A fuel gas, with
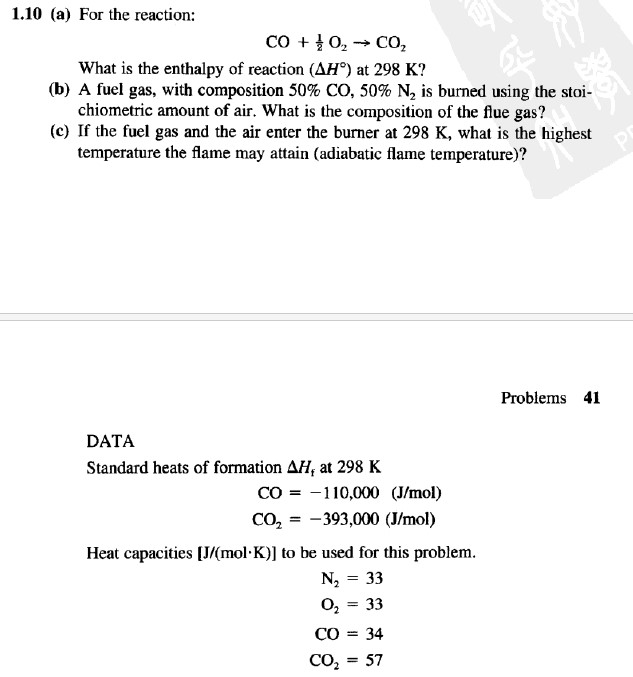
1.10 (a) For the reaction: CO + }O, C, What is the enthalpy of reaction (AH) at 298 K? (b) A fuel gas, with composition 50% CO, 50% N2 is burned using the stoi- chiometric amount of air. What is the composition of the flue gas? (c) If the fuel gas and the air enter the burner at 298 K, what is the highest temperature the flame may attain (adiabatic flame temperature)? DATA Standard heats of formation AH, at 298 K CO = 110,000 (J/mol) CO = -393,000 (J/mol) Heat capacities [J/(molK)] to be used for this problem. N = 33 O = 33 CO = 34 CO = 57 Problems 41
Step by Step Solution
There are 3 Steps involved in it
Combustion of CO and Air Enthalpy Flue Gas and Adiabatic Flame Temperature This problem explores the combustion of carbon monoxide CO with air and cal... View full answer

Get step-by-step solutions from verified subject matter experts


