Question: 12. The reaction PCls (g) = PC13 (g) + Cl2 (g) has been studied by mixing one mole of PC13 and one mole of Cl2
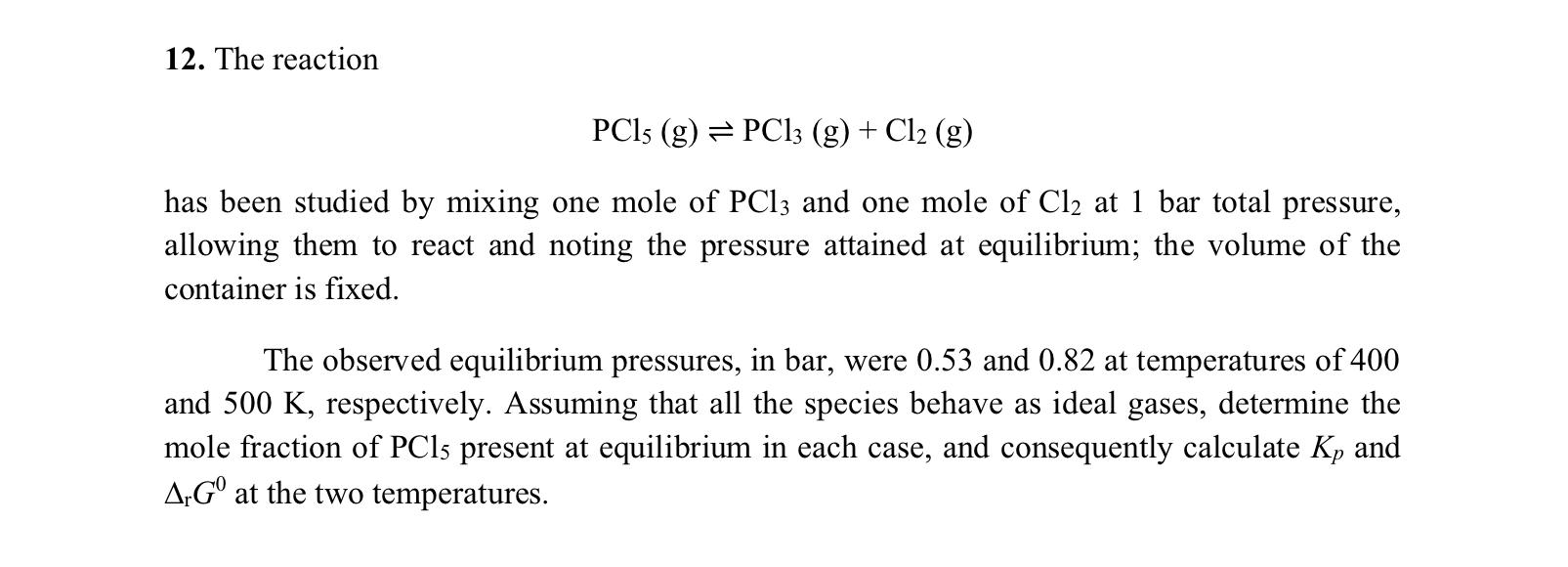
12. The reaction PCls (g) = PC13 (g) + Cl2 (g) has been studied by mixing one mole of PC13 and one mole of Cl2 at 1 bar total pressure, allowing them to react and noting the pressure attained at equilibrium; the volume of the container is fixed. The observed equilibrium pressures, in bar, were 0.53 and 0.82 at temperatures of 400 and 500 K, respectively. Assuming that all the species behave as ideal gases, determine the mole fraction of PC15 present at equilibrium in each case, and consequently calculate Kp and ArG at the two temperatures
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


