Question: 19. * Pt. A: Using curved-arrow notation to show the movement of electrons, draw all of the significant resonance structures for the following molecules. a)
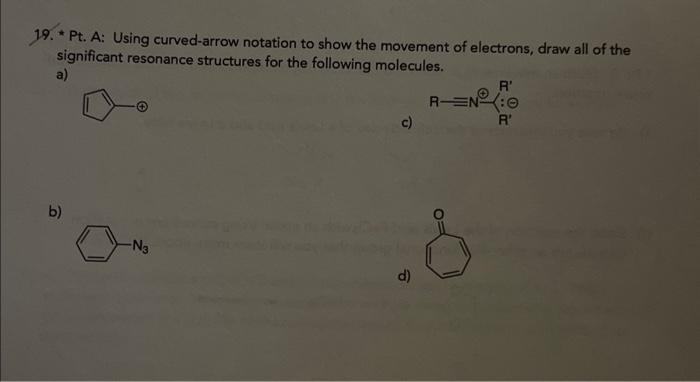

19. * Pt. A: Using curved-arrow notation to show the movement of electrons, draw all of the significant resonance structures for the following molecules. a) c) b) - Pt, B: Electron movements in acid base reactions can be represented with the same curvedarrow notation. a) Be sure you understand the defined roles of a Lewis acid and a Lewis base. b) Show the reaction of the Lewis acid FeCla with an acotal using curved-arrow notation (acetals are an ether-like functional group that you will leam more about in C342). 00+FeCl3 c) What orbitals were involved in the chemistry you just described? [You should give the orbital in terms of its hybridization, the independent property of whether it is filled/halffilled/empty, and its relative energy (e.g, highest energy filled orbital). Do you think certain types of orbitals are likely to be involved in Lewis acid-base interactions? d) Now show how one of the C.O bonds is weakened allowing the methyl-bearing carbon to be attacked by a nucleophile
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


