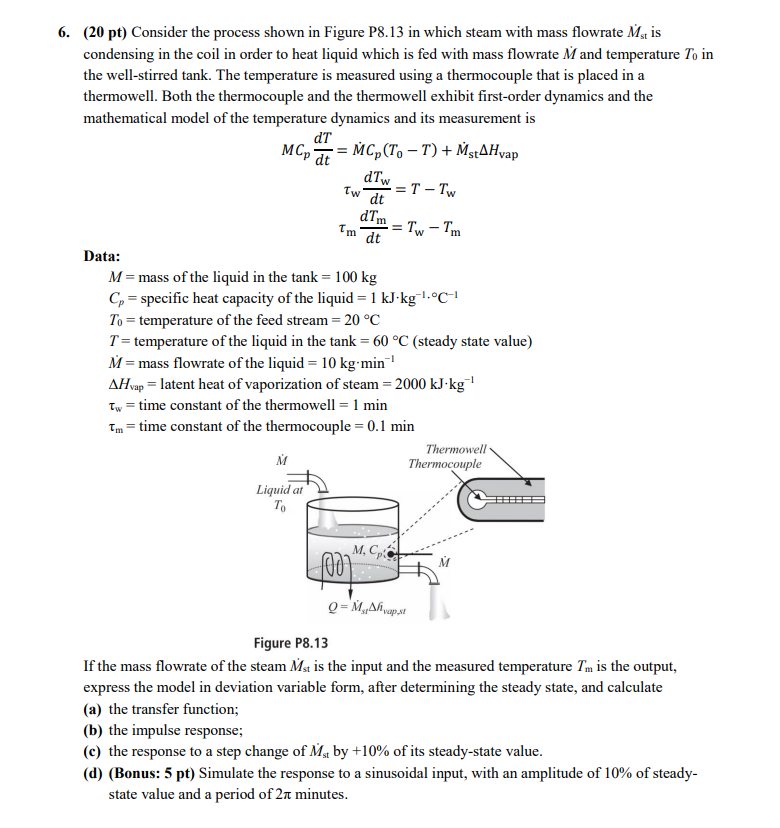
Question: ( 2 0 pt ) Consider the process shown in Figure P 8 . 1 3 in which steam with mass flowrate M s t
pt Consider the process shown in Figure P in which steam with mass flowrate is
condensing in the coil in order to heat liquid which is fed with mass flowrate and temperature in
the wellstirred tank. The temperature is measured using a thermocouple that is placed in a
thermowell. Both the thermocouple and the thermowell exhibit firstorder dynamics and the
mathematical model of the temperature dynamics and its measurement is
Data:
mass of the liquid in the tank
specific heat capacity of the liquid
temperature of the feed stream
temperature of the liquid in the tank steady state value
mass flowrate of the liquid
latent heat of vaporization of steam
time constant of the thermowell min
time constant of the thermocouple min
Figure P
If the mass flowrate of the steam is the input and the measured temperature is the output,
express the model in deviation variable form, after determining the steady state, and calculate
a the transfer function;
b the impulse response;
c the response to a step change of by of its steadystate value.
dBonus: pt Simulate the response to a sinusoidal input, with an amplitude of of steady
state value and a period of minutes.
please solve it not just explaination
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


