Question: ( 2 5 p t s ) The gas phase reaction 2 A 2 B + C with the rate law of - r A
The gas phase reaction with the rate law of takes place in two separate reactors in two different experiments. In the first experiment, conversion is achieved when pure A is fed to a PFR with at In the second experiment conversion is achieved when a mixture of A and inert is fed to a with at Calculate the activation energy in
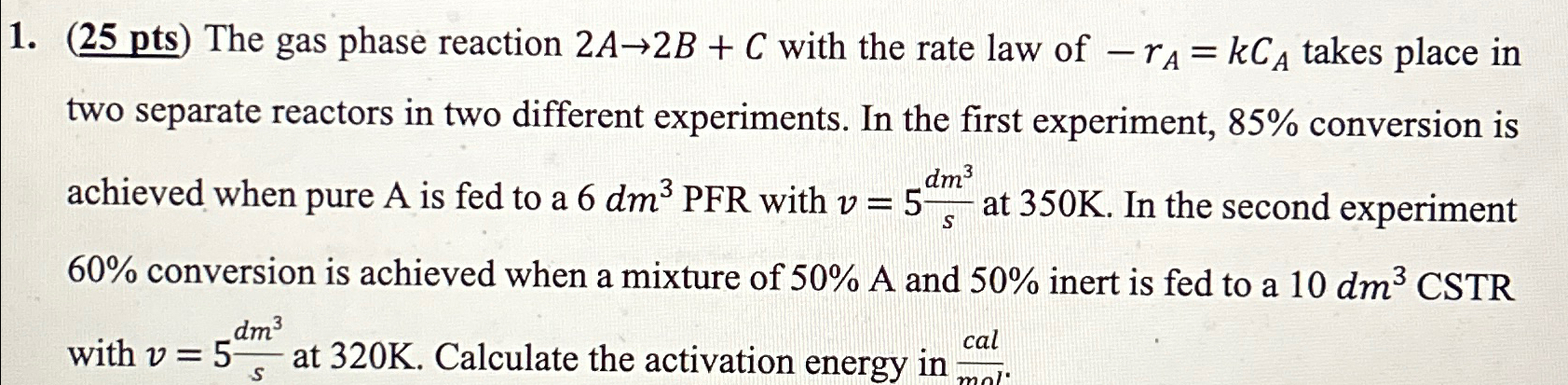
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


