Question: 2. Phosphate buffer a. 100 mM potassium-phosphate, pH 5.0 b. 100 mM potassium-phosphate, pH 7.0 Note: H3PO4 is a triprotic acid with pK 1 =
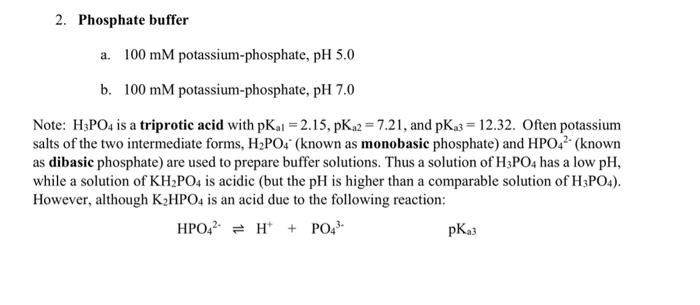
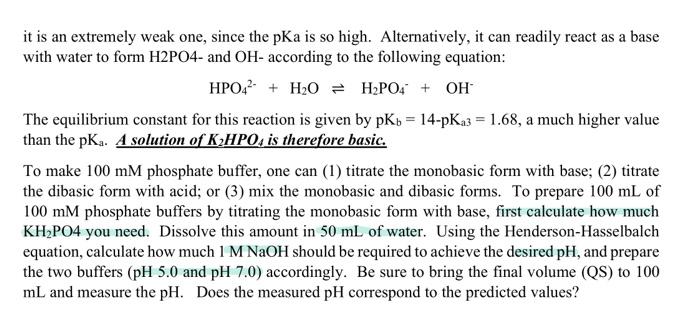
2. Phosphate buffer a. 100 mM potassium-phosphate, pH 5.0 b. 100 mM potassium-phosphate, pH 7.0 Note: H3PO4 is a triprotic acid with pK 1 = 2.15, PK 2 = 7.21, and pK 3 = 12.32. Often potassium salts of the two intermediate forms, H2PO4 (known as monobasic phosphate) and HPO 2 (known as dibasic phosphate) are used to prepare buffer solutions. Thus a solution of H3PO4 has a low pH, while a solution of KH2PO4 is acidic (but the pH is higher than a comparable solution of H3PO4). However, although K2HPO4 is an acid due to the following reaction: HPO42 = H + PO43- PK3 it is an extremely weak one, since the pKa is so high. Alternatively, it can readily react as a base with water to form H2PO4- and OH- according to the following equation: HPO42- + H2O = H2PO4 + OH The equilibrium constant for this reaction is given by pKb = 14-pKa3 = 1.68, a much higher value than the pK. A solution of K2HPO, is therefore basic. To make 100 mM phosphate buffer, one can (1) titrate the monobasic form with base; (2) titrate the dibasic form with acid; or (3) mix the monobasic and dibasic forms. To prepare 100 mL of 100 mM phosphate buffers by titrating the monobasic form with base, first calculate how much KH2PO4 you need. Dissolve this amount in 50 mL of water. Using the Henderson-Hasselbalch equation, calculate how much 1 M NaOH should be required to achieve the desired pH, and prepare the two buffers (pH 5.0 and pH 7.0) accordingly. Be sure to bring the final volume (QS) to 100 mL and measure the pH. Does the measured pH correspond to the predicted values
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


