Question: 2. Write out but don't solve the rate expression for the following reactions, assuming they are elementary. You may define a generic rate constant, ki
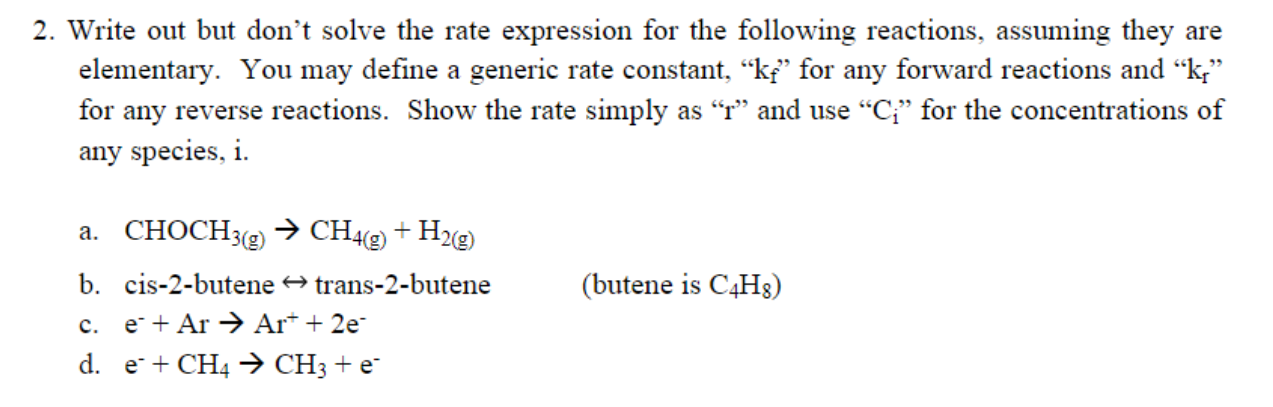
2. Write out but don't solve the rate expression for the following reactions, assuming they are elementary. You may define a generic rate constant, ki for any forward reactions and k; for any reverse reactions. Show the rate simply as and use C for the concentrations of any species, i. a. CHOCH3(g) CH49) + H2(g) b. cis-2-butene + trans-2-butene c. e* + Ar Ar+ + 2e d. e+ CH4 + CH3 + e- (butene is C4H8)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


