Question: 3 . 3 4 The important chemical intermediate acetaldehyde can be catalytically produced from partial oxidation of ethane. The primary reaction is C 2 H
The important chemical intermediate acetaldehyde can be catalytically produced from partial oxidation of ethane. The primary reaction is
However, there are a number of side reactions which also occur to a significant degree:
Problems
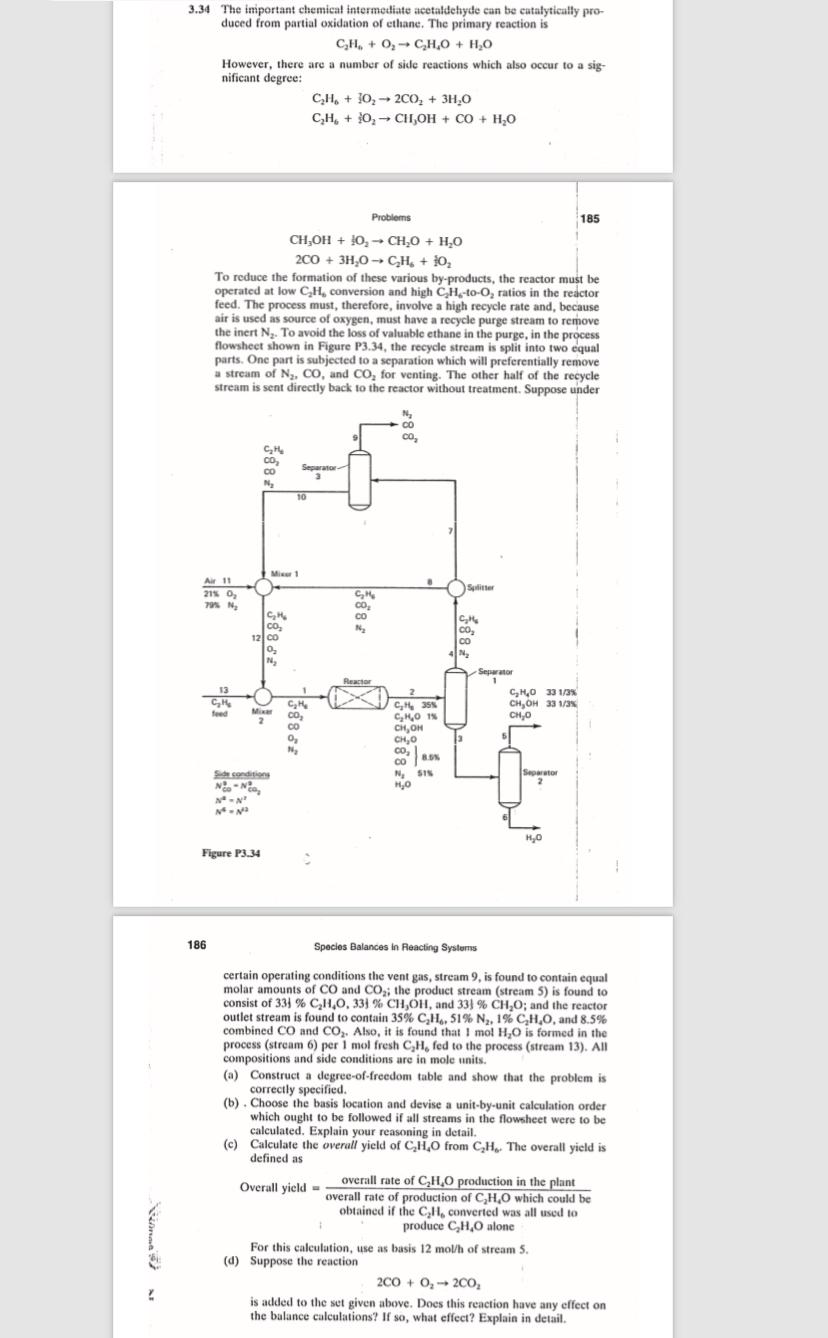
To reduce the formation of these various byproducts, the reactor must be operated at low conversion and high to ratios in the reactor feed. The process must, therefore, involve a high recycle rate and, because air is used as source of oxygen, must have a recycle purge stream to remove the inert To avoid the loss of valuable ethane in the purge, in the process flowshect shown in Figure P the recycle stream is split into two equal parts. One part is subjected to a separation which will preferentially remove a stream of and for venting. The other half of the recycle stream is sent directly back to the reactor without treatment. Suppose under
Species Balances in Reacting Systerns
certain operating conditions the vent gas, stream is found to contain equal molar amounts of and ; the product stream stream is found to consist of and ; and the reactor outlet stream is found to contain and combined and Also, it is found that is formed in the process stream per mol fresh fed to the process stream All compositions and side conditions are in mole units.
a Construct a degreeoffreedom table and show that the problem is correctly specified.
b Choose the basis location and devise a unitbyunit calculation order which ought to be followed if all streams in the flowsheet were to be calculated. Explain your reasoning in detail.
c Calculate the overall yield of from The overall yield is defined as
Overall yield
For this calculation, use as basis of stream
d Suppose the reaction
y
is added to the set given above. Does this reaction have any effect on the balance calculations? If so what effect? Explain in detail.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


