Question: 3. For each substance below, identify the most acidic hydrogen. For the following substances, write equations showing their dissociation in water. Identify the conjugate base.
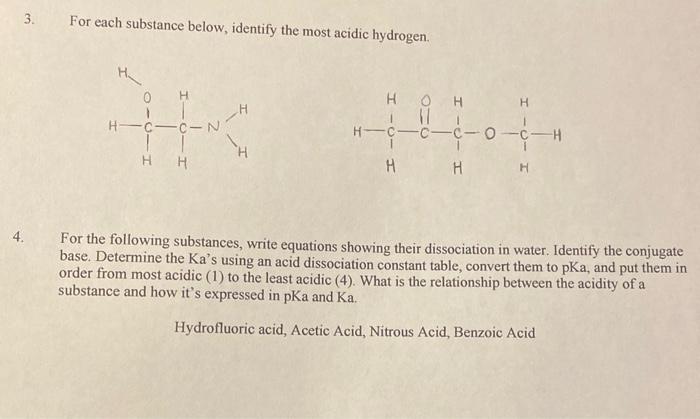
3. For each substance below, identify the most acidic hydrogen. For the following substances, write equations showing their dissociation in water. Identify the conjugate base. Determine the Ka's using an acid dissociation constant table, convert them to pKa, and put them in order from most acidic (1) to the least acidic (4). What is the relationship between the acidity of a substance and how it's expressed in pKa and Ka. Hydrofluoric acid, Acetic Acid, Nitrous Acid, Benzoic Acid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


