Question: 3. Which would be most soluble in C6H14 (hexane)? Explain why! H2NCH2CH2CH2NH2orCH3CH2CH2CH2NH2
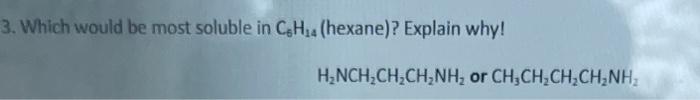
3. Which would be most soluble in C6H14 (hexane)? Explain why! H2NCH2CH2CH2NH2orCH3CH2CH2CH2NH2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


