Question: 36) For the equation: 2 CH2Cl2 (g) CH4 (g) + CCl4 (g), you start with 0.25 M of CH2Cl2 and it has a Kc
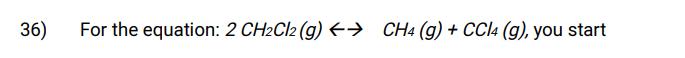

36) For the equation: 2 CH2Cl2 (g) CH4 (g) + CCl4 (g), you start with 0.25 M of CH2Cl2 and it has a Kc value of 0.84. What are the equilibrium concentrations of all species?
Step by Step Solution
3.50 Rating (147 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


