Question: 5. Consider the balanced equation below in which 10.86 grams of PCl5 reacts with 13.58 grams of AsF3.3(30.94)+s(3s.45)=270.16 3PCl5+5AsF33PF5+5AsCl3s(74.92)+3(18.90)=431.54 What is the excess reactant? A.
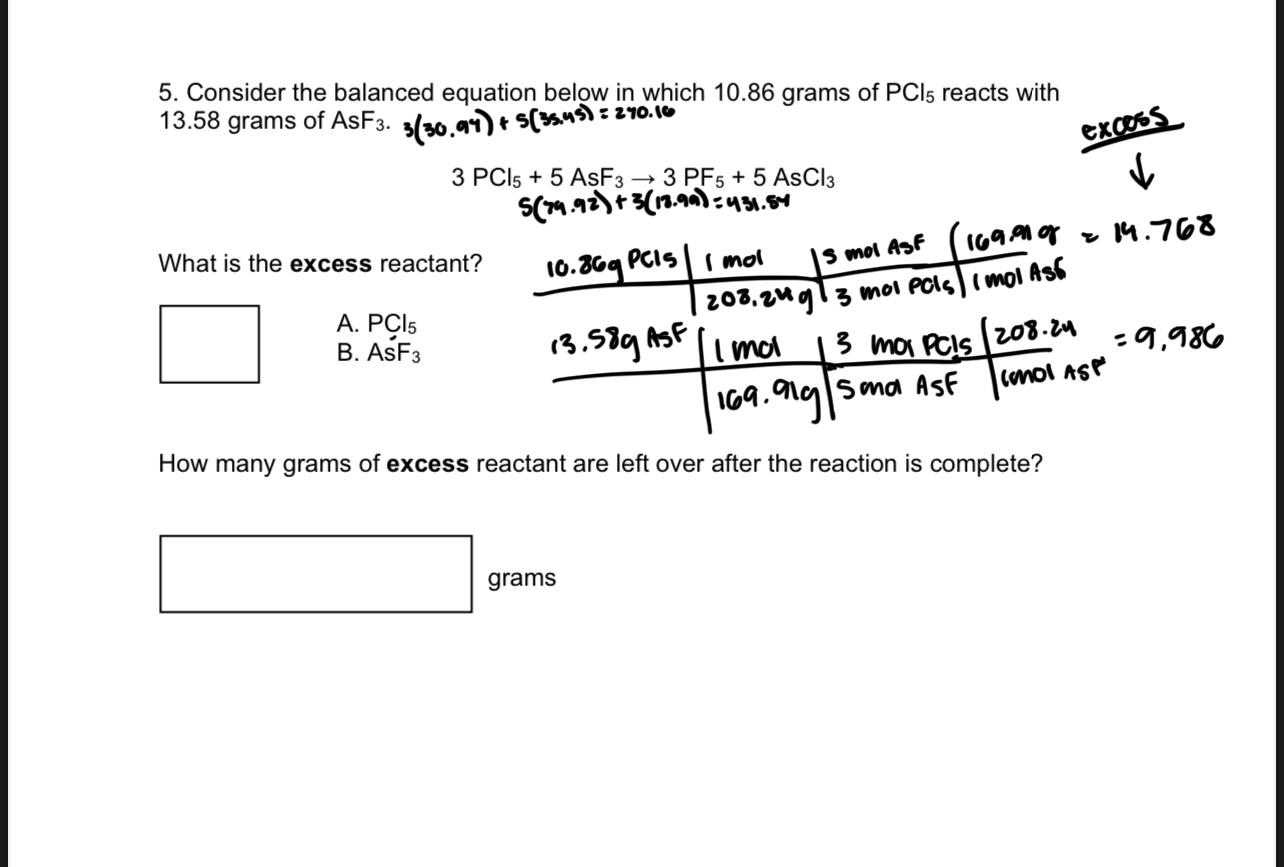
5. Consider the balanced equation below in which 10.86 grams of PCl5 reacts with 13.58 grams of AsF3.3(30.94)+s(3s.45)=270.16 3PCl5+5AsF33PF5+5AsCl3s(74.92)+3(18.90)=431.54 What is the excess reactant? A. PCCl5 B. AsF3 How many grams of excess reactant are left over after the reaction is complete? grams
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


