Question: Given the data in the table below, AHrxn for the reaction 2Ag25 (s) + 02 (8)+ 2Ag20 (6) + 25 (s) is kJ. Substance
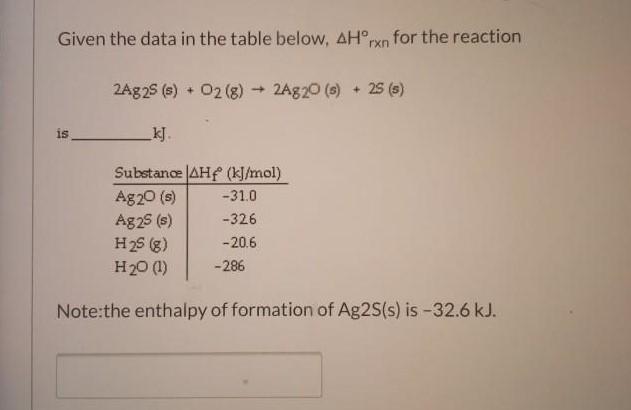
Given the data in the table below, AHrxn for the reaction 2Ag25 (s) + 02 (8)+ 2Ag20 (6) + 25 (s) is kJ. Substance AHf (kJ/mol) Ag20 (s) Ag25 (s) H25 (g) H20 (1) -31.0 -326 -20.6 -286 Note:the enthalpy of formation of Ag2S(s) is-32.6 kJ.
Step by Step Solution
There are 3 Steps involved in it
Given Data Reaction 2Ag2S s O2 g ightarrow 2Ag2O s 2S s Standard Enthalpies of Forma... View full answer

Get step-by-step solutions from verified subject matter experts


