Question: A buret contains an unknown solution. Initially the buret was filled with the solution so that it read 11.84 mL and the balance below read
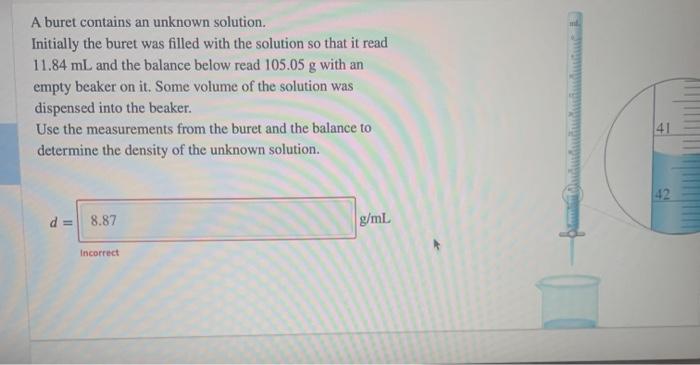
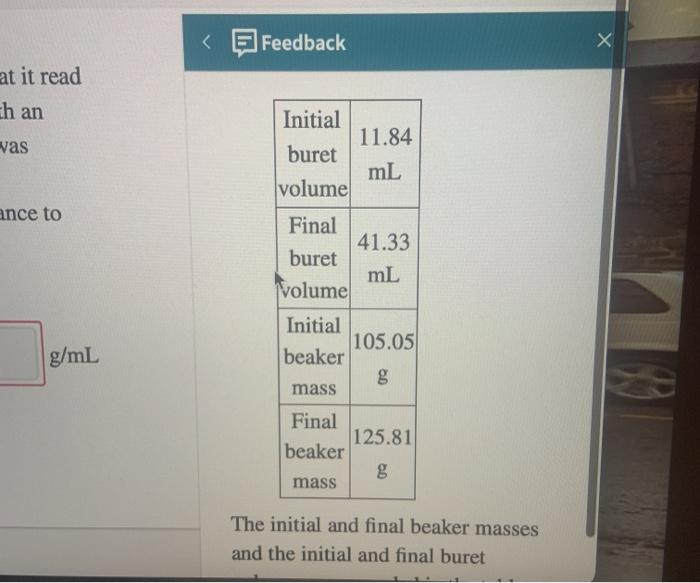
A buret contains an unknown solution. Initially the buret was filled with the solution so that it read 11.84 mL and the balance below read 105.05 g with an empty beaker on it. Some volume of the solution was dispensed into the beaker. Use the measurements from the buret and the balance to determine the density of the unknown solution. 41 42 d = 8.87 g/ml Incorrect
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


