Question: A small reaction bomb fitted with a sensitive pressure measuring device is flushed out and filled with pure dimethyl ether at 140'C and 760
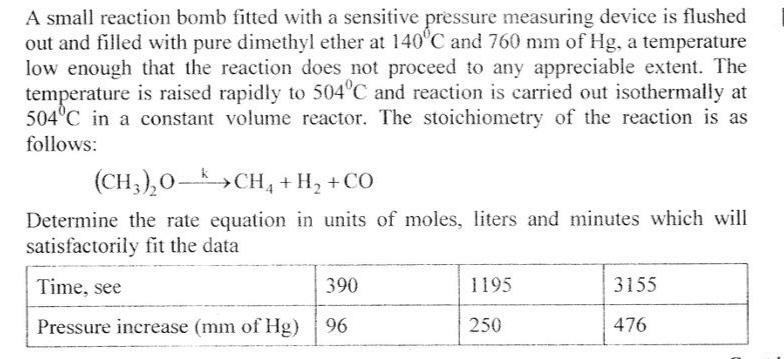
A small reaction bomb fitted with a sensitive pressure measuring device is flushed out and filled with pure dimethyl ether at 140'C and 760 mm of Hg, a temperature low enough that the reaction does not proceed to any appreciable extent. The temperature is raised rapidly to 504C and reaction is carried out isothermally at 504C in a constant volume reactor. The stoichiometry of the reaction is as follows: (CH,),0- (CH,),0->CH, + H, +CO Determine the rate equation in units of moles, liters and minutes which will satisfactorily fit the data 390 1195 3155 Time, see Pressure increase (mm of Hg) 476 96 250
Step by Step Solution
3.30 Rating (156 Votes )
There are 3 Steps involved in it
To determine the rate equation we follow these steps Step 1 Understanding the Reaction and Data The reaction given is textCH32textO ightarrow textCH4 ... View full answer

Get step-by-step solutions from verified subject matter experts


