Question: A solution containing an unknown cation reacts spontaneously with both zinc (8ox= +0.76 V) and copper (Eox = -0.34 V). The unknown cation is [A]
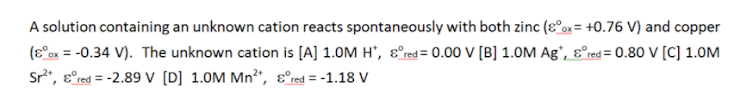
A solution containing an unknown cation reacts spontaneously with both zinc (8ox= +0.76 V) and copper (Eox = -0.34 V). The unknown cation is [A] 1.0M H*, & red=0.00 V [B] 1.0M Ag , & red = 0.80 V (C) 1.0M Sr?*, & red=-2.89 V [D] 1.0M Mn2t, & red=-1.18 V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


