Question: A weight is added to a piston so that the volume of the gas inside the container is reduced from 2.5 L to 1.0
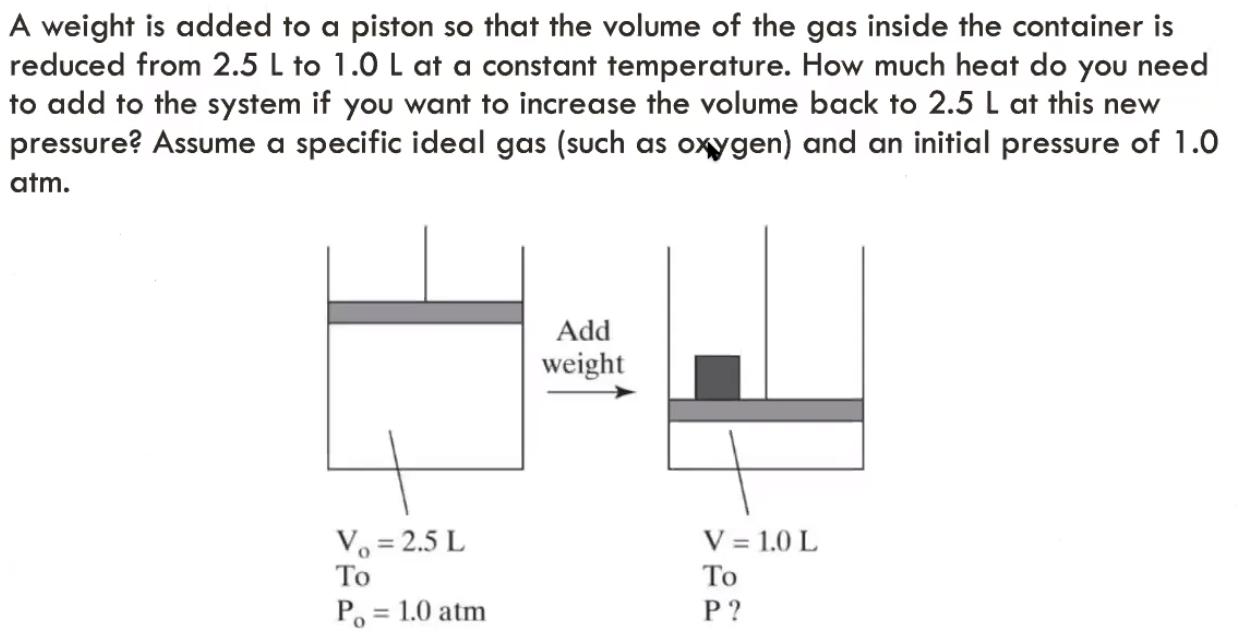
A weight is added to a piston so that the volume of the gas inside the container is reduced from 2.5 L to 1.0 L at a constant temperature. How much heat do you need to add to the system if you want to increase the volume back to 2.5 L at this new pressure? Assume a specific ideal gas (such as oxygen) and an initial pressure of 1.0 atm. Add weight V = 1.0 L Vo = 2.5 L To P. = 1.0 atm P ? %3D
Step by Step Solution
★★★★★
3.52 Rating (159 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


