Question: above is my data from the lab. how do i answer the theoretical yield and the 3 questions? Calculations Mass of empty crucible and cover
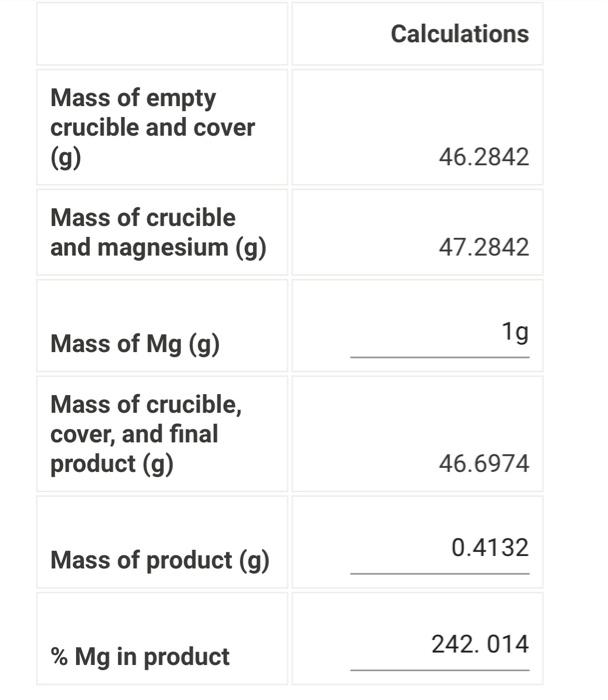

Calculations Mass of empty crucible and cover (g) 46.2842 Mass of crucible and magnesium (g) 47.2842 Mass of Mg(g) 1g Mass of crucible, cover, and final product (g) 46.6974 Mass of product (g) 0.4132 %Mg in product 242. 014 (0.5pts) What is the theoretical \% Mg in MgO ? (0.5pts) What is the theoretical \% Mg in Mg3N2 ? (0.5pts) Based on your comparison of the %Mg in your product (as calculated above) with the %Mg in the two possible products, what is the identity of your product Chnnes Choose... MgO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


