Question: Ammonia (NH3) will react with a strong acid, such as hydronium (H30*), to give an ammonium ion, as shown below. This type of process is
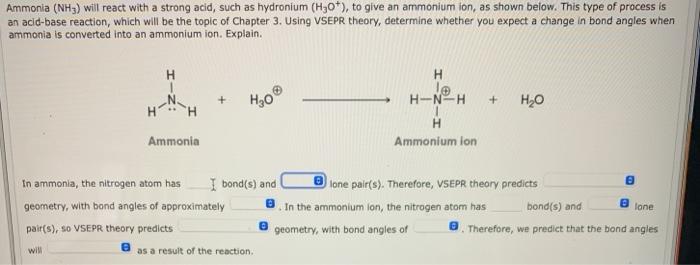
Ammonia (NH3) will react with a strong acid, such as hydronium (H30*), to give an ammonium ion, as shown below. This type of process is an acid-base reaction, which will be the topic of Chapter 3. Using VSEPR theory, determine whether you expect a change in bond angles when ammonia is converted into an ammonium ion. Explain. H H + + ,0 H-N-H H Ammonium ion Ammonia In ammonia, the nitrogen atom has I bond(s) and lone pair(s). Therefore, VSEPR theory predicts geometry, with bond angles of approximately In the ammonium lon, the nitrogen atom has bond(s) and pair(s), so VSEPR theory predicts geometry, with bond angles of Therefore, we predict that the bond angles lone will e as a result of the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


