Question: An aqueous solution of reactant A with initial concentration Cao is charged into a batch reactor. The reactant A undergoes the following series reactions in
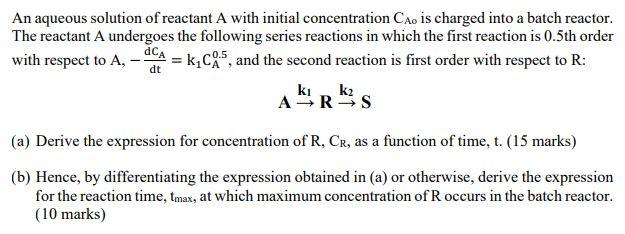
An aqueous solution of reactant A with initial concentration Cao is charged into a batch reactor. The reactant A undergoes the following series reactions in which the first reaction is 0.5th order with respect to A, = kc 5, and the second reaction is first order with respect to R: dt ki, k2 ARS (a) Derive the expression for concentration of R, Cr, as a function of time, t. (15 marks) (b) Hence, by differentiating the expression obtained in (a) or otherwise, derive the expression for the reaction time, tmax, at which maximum concentration of Roccurs in the batch reactor. (10 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


