Question: An aqueous solution of reactant A with initial concentration Cao is charged into a batch reactor. The reactant A undergoes the following series reactions in
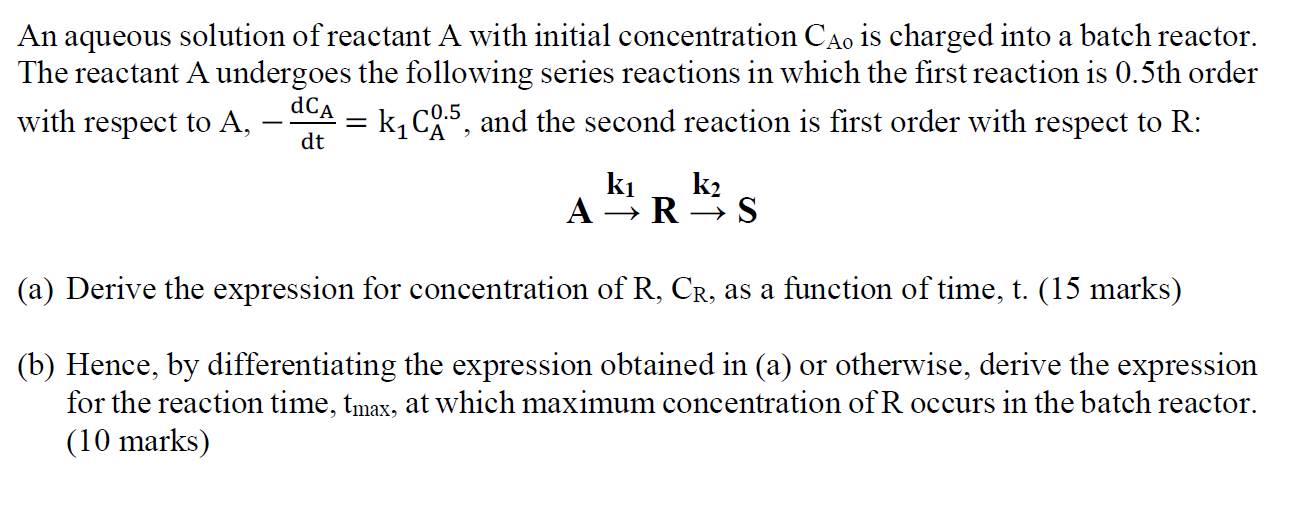
An aqueous solution of reactant A with initial concentration Cao is charged into a batch reactor. The reactant A undergoes the following series reactions in which the first reaction is 0.5th order da with respect to A, = k1CA5, and the second reaction is first order with respect to R: ARRks k2 dt (a) Derive the expression for concentration of R, CR, as a function of time, t. (15 marks) (b) Hence, by differentiating the expression obtained in (a) or otherwise, derive the expression for the reaction time, tmax, at which maximum concentration of R occurs in the batch reactor. (10 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


