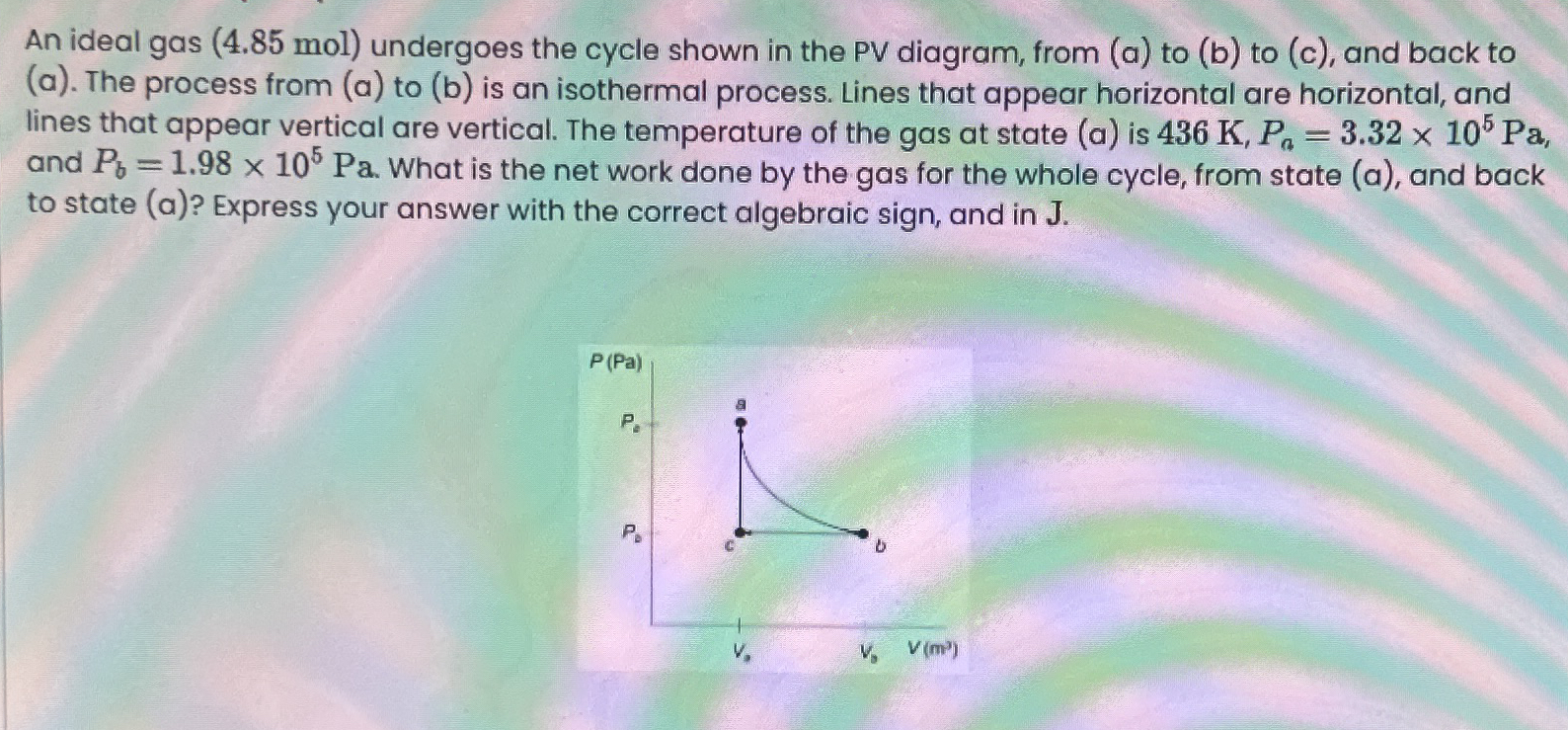
Question: An ideal gas ( 4 . 8 5 mol ) undergoes the cycle shown in the PV diagram, from ( a ) to ( b
An ideal gas mol undergoes the cycle shown in the PV diagram, from a to b to c and back to a The process from a to b is an isothermal process. Lines that appear horizontal are horizontal, and lines that appear vertical are vertical. The temperature of the gas at state a is and What is the net work done by the gas for the whole cycle, from state a and back to state a Express your answer with the correct algebraic sign, and in J
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


