Question: The enthalpy of fusion of Ice is 6.02 k.J mol-. The heat capacity of H2O is 4.18J g-1K-1. What is the smallest number of
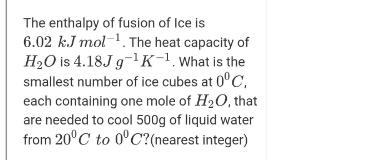
The enthalpy of fusion of Ice is 6.02 k.J mol-. The heat capacity of H2O is 4.18J g-1K-1. What is the smallest number of ice cubes at 0C, each containing one mole of H20, that are needed to cool 500g of liquid water from 20C to 0C?(nearest integer)
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
msTnH fus Where mmasssspecific heat ... View full answer

Get step-by-step solutions from verified subject matter experts


