Question: Balancing Oxidation-Reduction Reactions Use the References to access important values if needed for this question. When the following equation is balanced properly under acidic conditions,
Balancing Oxidation-Reduction Reactions 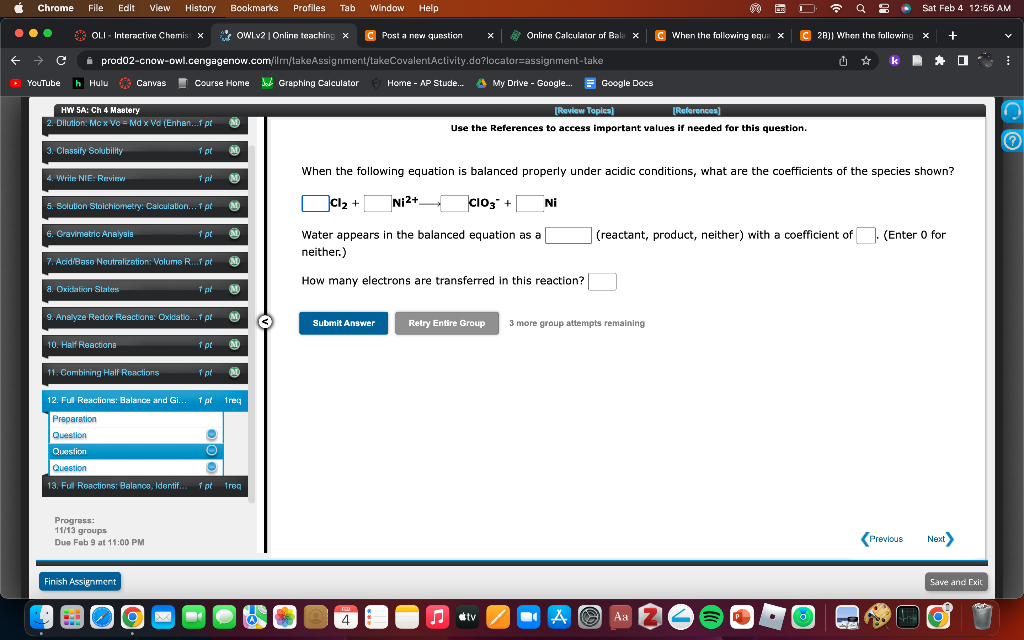
Use the References to access important values if needed for this question. When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? Cl2+Ni2+ClO3+Ni Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of . (Enter ofor neither.) How many electrons are transferred in this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


