Question: can some body explain me how to do this In k = -Ea + InA RT (2 points) In order to find the activation energy

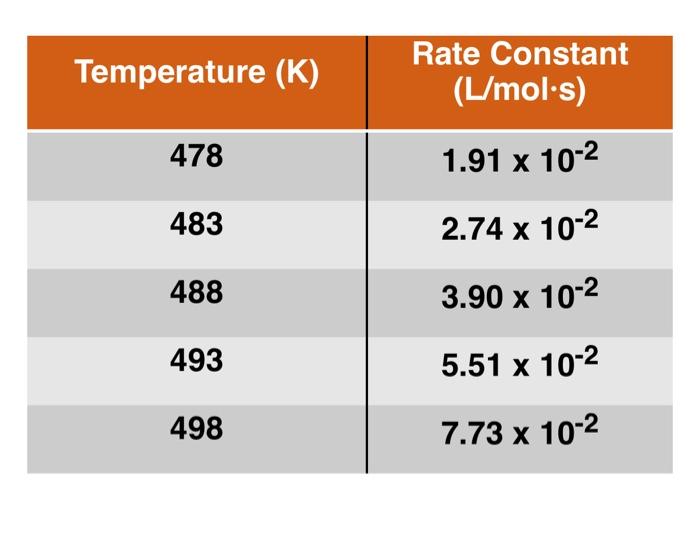
In k = -Ea + InA RT (2 points) In order to find the activation energy (E.) you will need to make a linear graph. What should be plotted on the y-axis? The x-axis? (2 points) Will the slope of such a line be equal to E.? If not, write an equation relating the slope (m) to E (2 points) Using the variable b to represent the y-intercept, write an equation relating b to A, the frequency factor. (3 points) Make an Arrhenius plot in MS Excel using the data below and determine the values of E, and A. Be sure that the graph you hand in is labeled and titled properly. (1 point) What is the value of R? for this data? What does it mean? Temperature (K) Rate Constant (L/mol.s) 478 1.91 x 10-2 483 2.74 x 10-2 488 3.90 x 10-2 493 5.51 x 10-2 498 7.73 x 10-2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


