Question: can someone help? I need help with this assignment. The following chemical reaction takes place in aqueous solution: CuBr2(aq)+(NH4)2S(aq)CuS(s)+2NH4Br(aq) Write the net ionic equation for
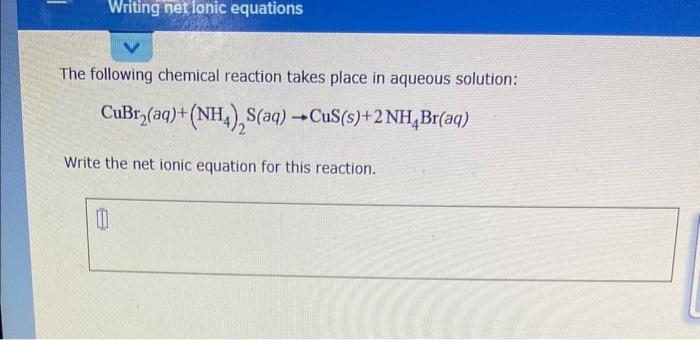
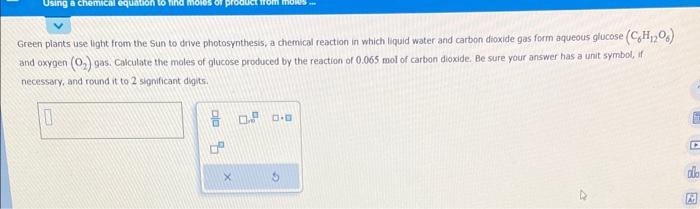
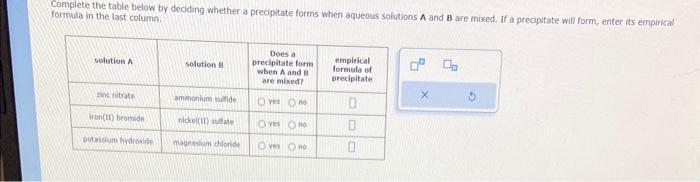
The following chemical reaction takes place in aqueous solution: CuBr2(aq)+(NH4)2S(aq)CuS(s)+2NH4Br(aq) Write the net ionic equation for this reaction. Greeri plants use light from the Sun to drive photosynthesis, a chemical reaction in which liquid water and carbon dioxide gas form aqueous glucose ( C6H12O6 ) and oxygen (O2) gas. Calculate the moles of glucose produced by the reaction of 0.065 mol of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to 2 sipmificant digits. Complete the table below by deoding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


