Question: chem help Current Attempt in Progress Naturally occurring magnesium is composed of 78.99% of 24Mg (atomic mass, 23.9850amu ), 10.00% of 25Mg (atomic mass, 24.9858
chem help
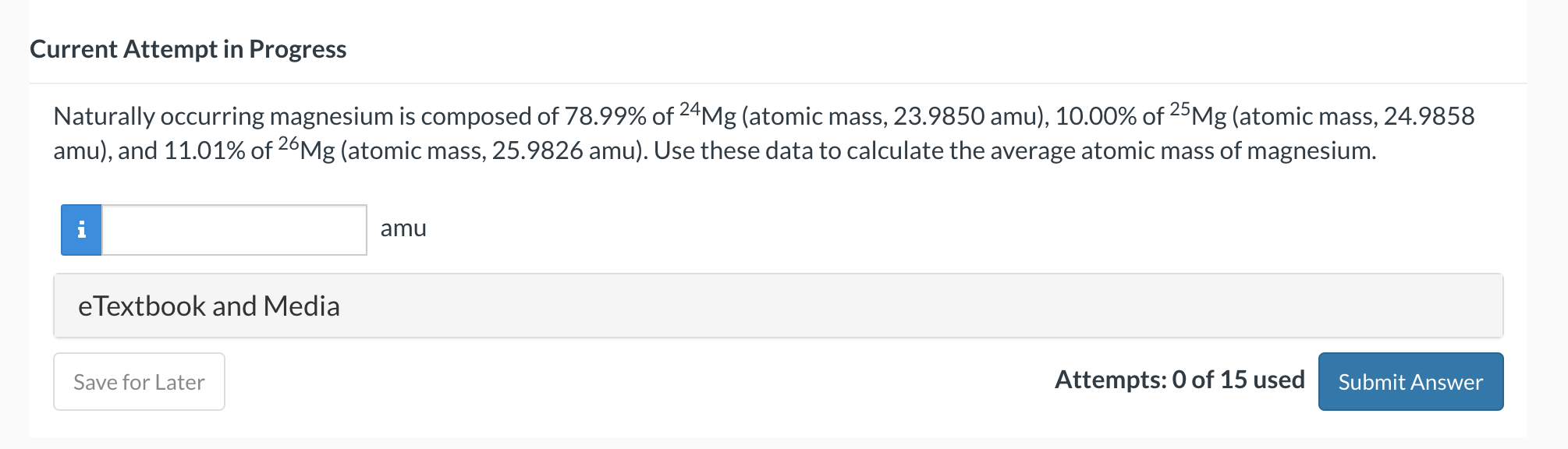
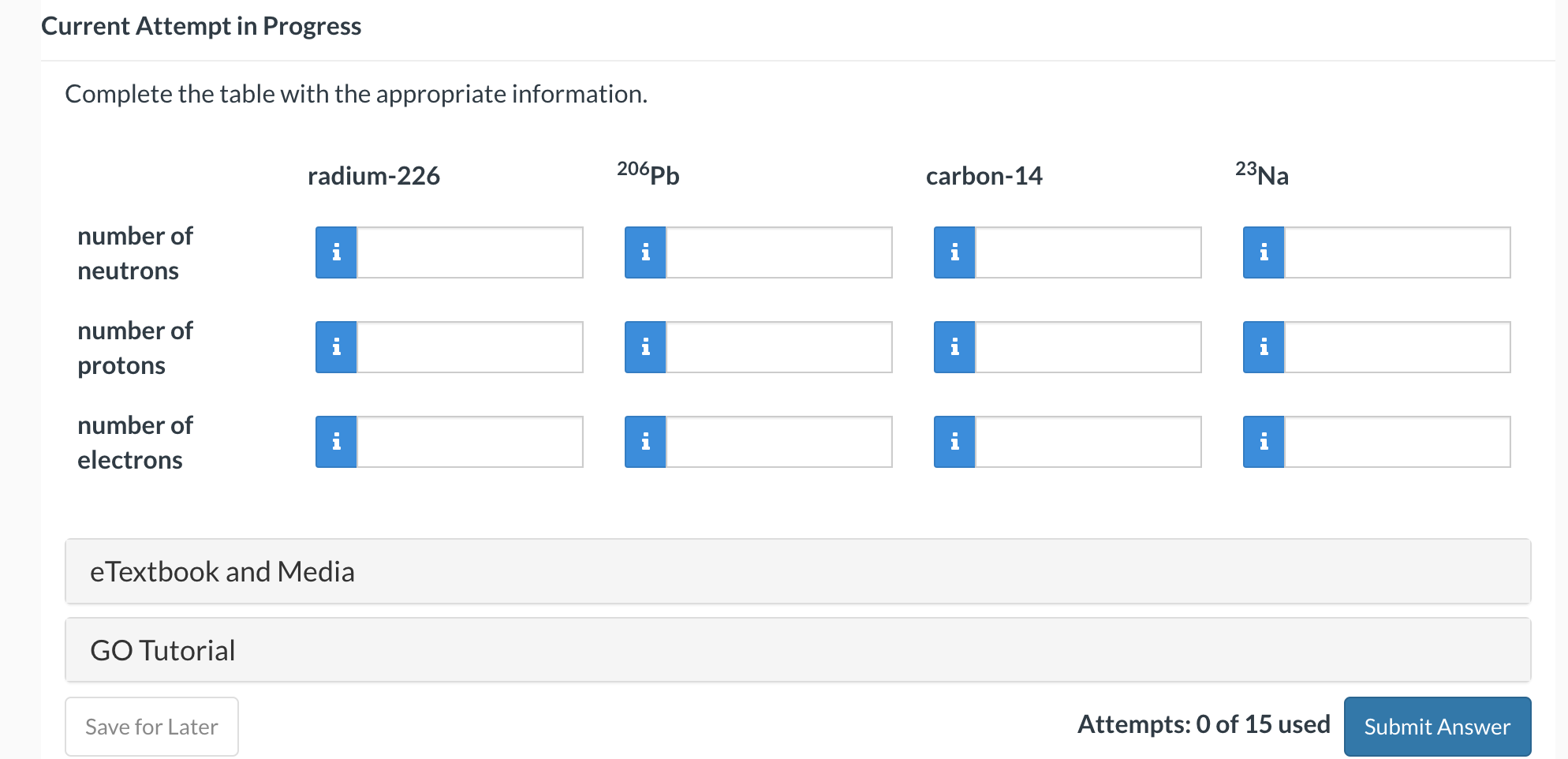
Current Attempt in Progress Naturally occurring magnesium is composed of 78.99% of 24Mg (atomic mass, 23.9850amu ), 10.00% of 25Mg (atomic mass, 24.9858 amu), and 11.01% of 26Mg (atomic mass, 25.9826amu ). Use these data to calculate the average atomic mass of magnesium. amu eTextbook and Media Attempts: 0 of 15 used Current Attempt in Progress Complete the table with the appropriate information. eTextbook and Media GO Tutorial Attempts: 0 of 15 used
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


