Question: Consider a 0.10 mol L-1 HNO2 (aq) acid solution having Ka 5.0 x 10-4 at %3D 25C. a) Write the acid dissociation equation. b)
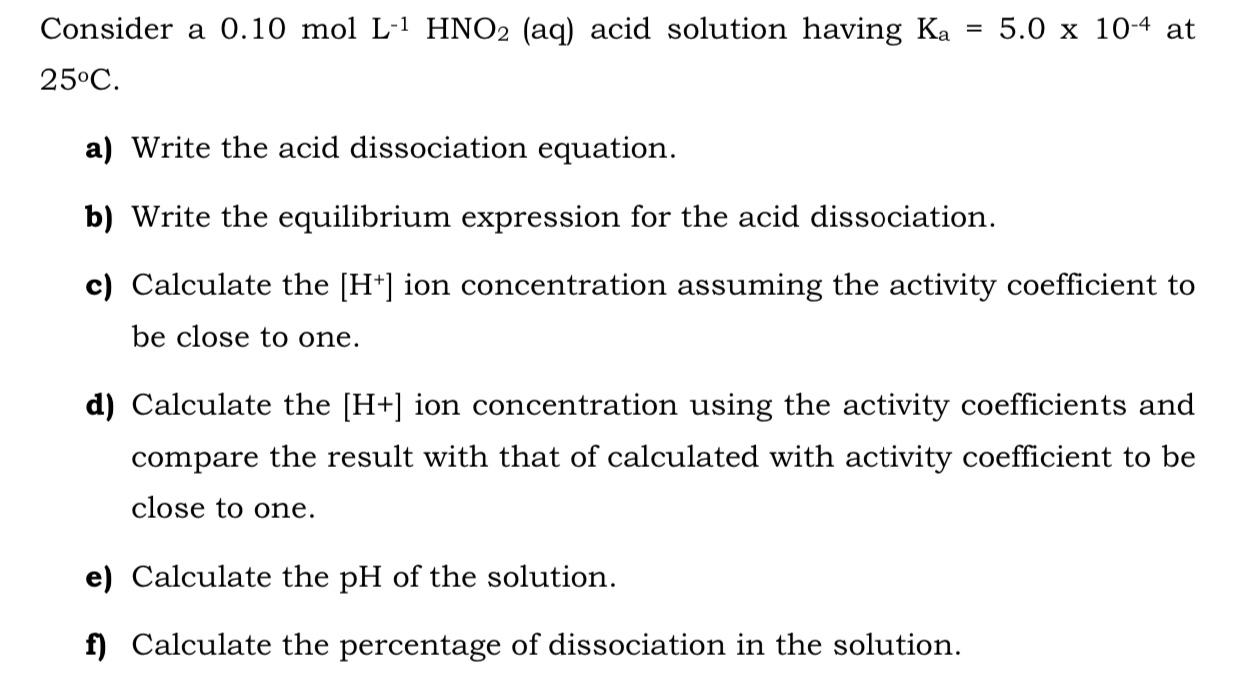
Consider a 0.10 mol L-1 HNO2 (aq) acid solution having Ka 5.0 x 10-4 at %3D 25C. a) Write the acid dissociation equation. b) Write the equilibrium expression for the acid dissociation. c) Calculate the [H*] ion concentration assuming the activity coefficient to be close to one. d) Calculate the [H+] ion concentration using the activity coefficients and compare the result with that of calculated with activity coefficient to be close to one. e) Calculate the pH of the solution. f) Calculate the percentage of dissociation in the solution.
Step by Step Solution
3.45 Rating (164 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


