Question: Consider heating solid water (ice) untii it becomes quid and then gas (steam) (Fgure.1). How much heat energy, in kilojoules, is required to convert 64.0g
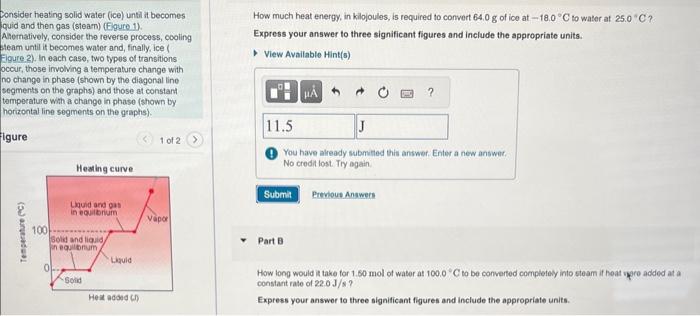

Consider heating solid water (ice) untii it becomes quid and then gas (steam) (Fgure.1). How much heat energy, in kilojoules, is required to convert 64.0g of ico at 18.0C to water at 25.0C ? A Aernatively, consider the reverse process, cooling Express your answer to three significant figures and include the appropriate units. beam until it beomes water and, finally, ice ( igure. 2). In each case, two types ol transitions occur, those involving a temperature change with no chango in phase (shown by the diagonal line segments on the graphs) and those at constart temperature with a change in phase (shown by horizontal line segments on the graphs). (1) You have already submitied this answer. Enter a new answet. No credit lost. Try again. Part 9 How long would it take for 1.60 mol of water at 100.0C to be converted completely into steam if heat upere added at a constant rate of 22.0J/s ? Express your answer to three significant figures and include the appropriate units. How long would it take tor 1.50mol of water at 100.0C to be converted completely into steam if heat were added at a constant rate of 22.0J/s ? Express your answer to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


