Question: What is the enthalpy of reaction for the following reaction: CH4(g) + 4Cl2(g) CCl4(g) + 4HCI(g) Bond Bond energy (kJ/mol) C-H 413 CI-CI 243
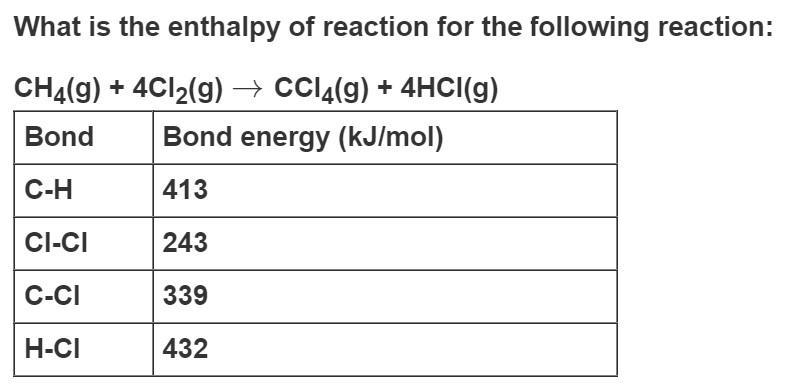
What is the enthalpy of reaction for the following reaction: CH4(g) + 4Cl2(g) CCl4(g) + 4HCI(g) Bond Bond energy (kJ/mol) C-H 413 CI-CI 243 C-CI 339 H-CI 432
Step by Step Solution
★★★★★
3.46 Rating (162 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Enthalpy of reac... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


