Question: Determine an ionic radius from unit cell dimensions. KCl crystallizes in a cubic unit cell with the NaCl structure, as represented in the following model.
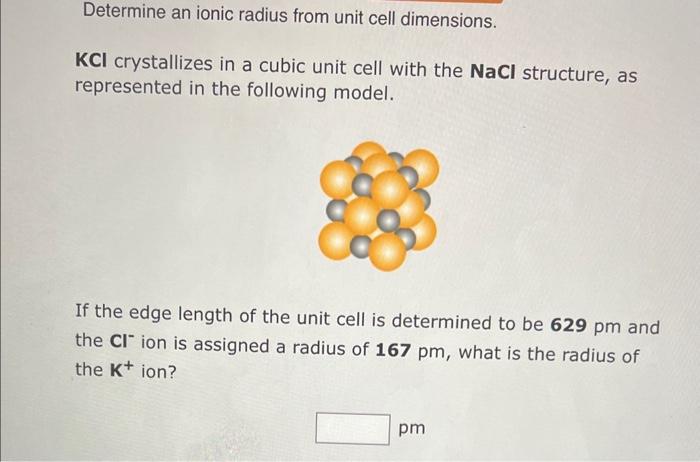
Determine an ionic radius from unit cell dimensions. KCl crystallizes in a cubic unit cell with the NaCl structure, as represented in the following model. If the edge length of the unit cell is determined to be 629pm and the Clion is assigned a radius of 167pm, what is the radius of the K+ion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


