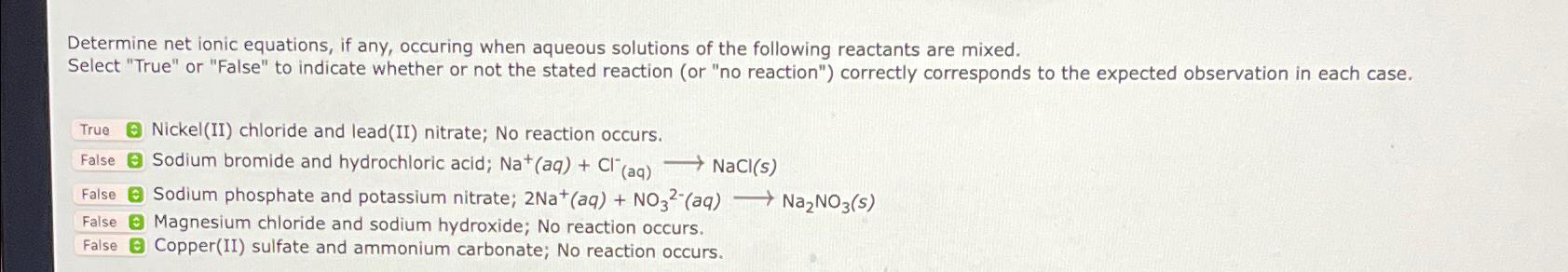
Question: Determine net ionic equations, if any, occuring when aqueous solutions of the following reactants are mixed. Select True or False to indicate whether or not
Determine net ionic equations, if any, occuring when aqueous solutions of the following reactants are mixed.
Select "True" or "False" to indicate whether or not the stated reaction or no reaction" correctly corresponds to the expected observation in each case.
NickelII chloride and leadII nitrate; No reaction occurs.
False Sodium bromide and hydrochloric acid; longrightarrowNaCl
False Sodium phosphate and potassium nitrate;
False Magnesium chloride and sodium hydroxide; No reaction occurs.
CopperII sulfate and ammonium carbonate; No reaction occurs.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


