Question: Dinitrogen monoxide, N2O, decompose into nitrogen, N2, and oxygen, O2, when heated. The initial rate of the reaction is 0.421M/s. What is the initial rate
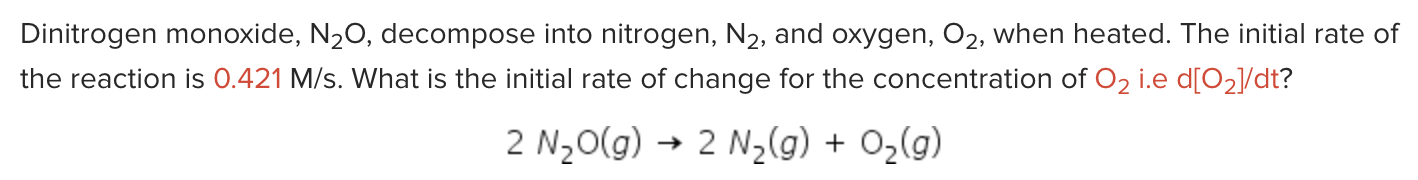
Dinitrogen monoxide, N2O, decompose into nitrogen, N2, and oxygen, O2, when heated. The initial rate of the reaction is 0.421M/s. What is the initial rate of change for the concentration of O2 i.e d[O2]/dt ? 2N2O(g)2N2(g)+O2(g)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


