Question: Dinitrogen monoxide, N2O, decompose into nitrogen, N2, and oxygen, O2, when heated. The initial rate of the reaction is 0.481M/s. What is the initial rate
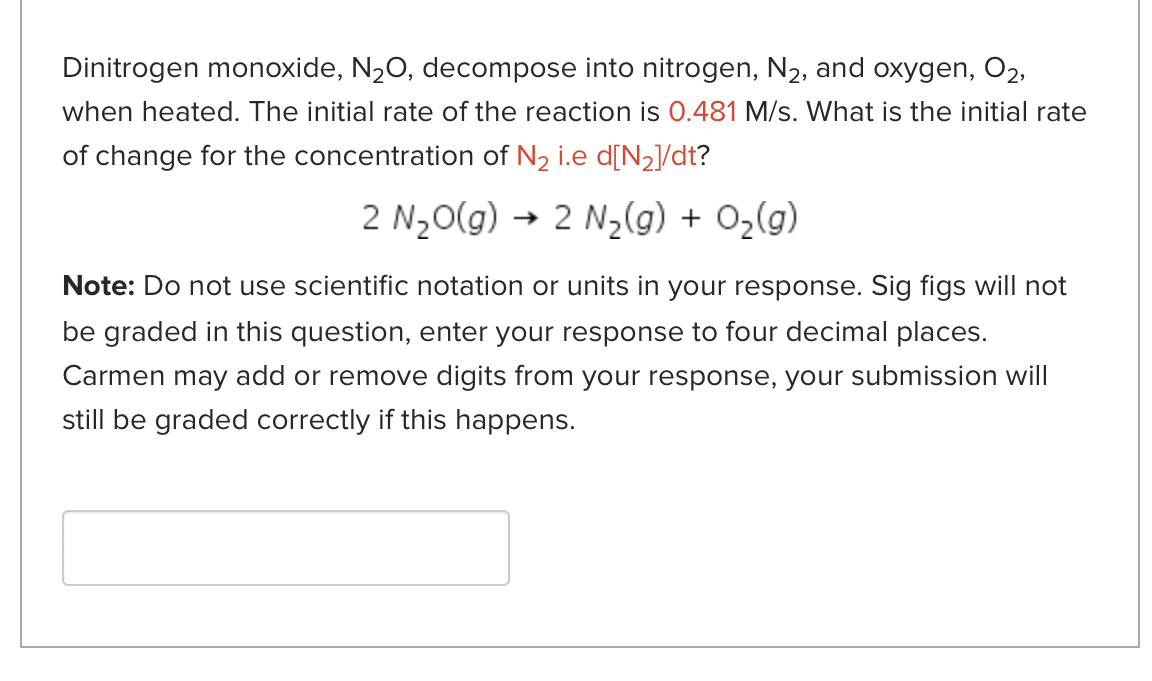
Dinitrogen monoxide, N2O, decompose into nitrogen, N2, and oxygen, O2, when heated. The initial rate of the reaction is 0.481M/s. What is the initial rate of change for the concentration of N2 i.e d[N2]/dt ? 2N2O(g)2N2(g)+O2(g) Note: Do not use scientific notation or units in your response. Sig figs will not be graded in this question, enter your response to four decimal places. Carmen may add or remove digits from your response, your submission will still be graded correctly if this happens
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


