Question: Every lone pair is shown, which structure has the incorrect formal charge? Choose the correct hybridization of every carbon molecule labeled D B D A
Every lone pair is shown, which structure has the incorrect formal charge?
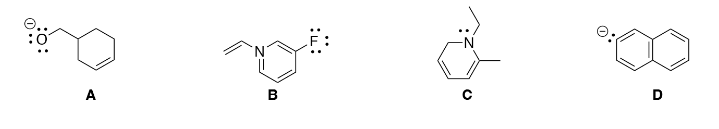
Choose the correct hybridization of every carbon molecule labeled
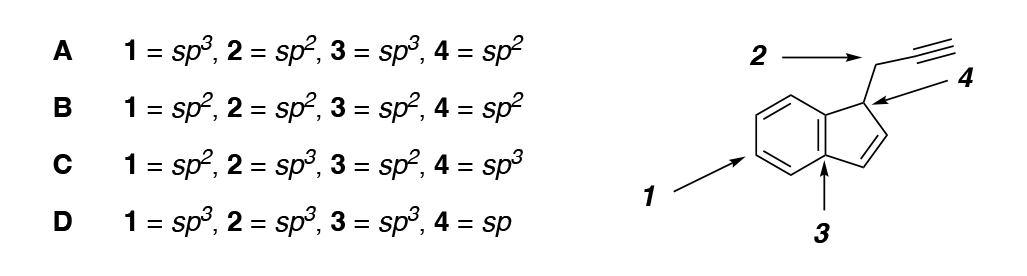
D B D A = 2 7 3 B - 1 = sp3, 2 = sp?, 3 = sp3, 4 = sp? 1 = spa, 2 = sp?, 3 = sp?, 4 = sp? 1 = sp?, 2 = sp3, 3 = sp?, 4 = sp3 1 = sp), 2 = sp3, 3 = sp, 4 = sp = 7 D = =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


