Question: example 5 and 6 are about Calculate the Equilibrium Constant and other are about Manipulating Equilibrium Constants. please the steps I need to do in

example 5 and 6 are about Calculate the Equilibrium Constant and other are about Manipulating Equilibrium Constants. please the steps I need to do in order solve! thank you
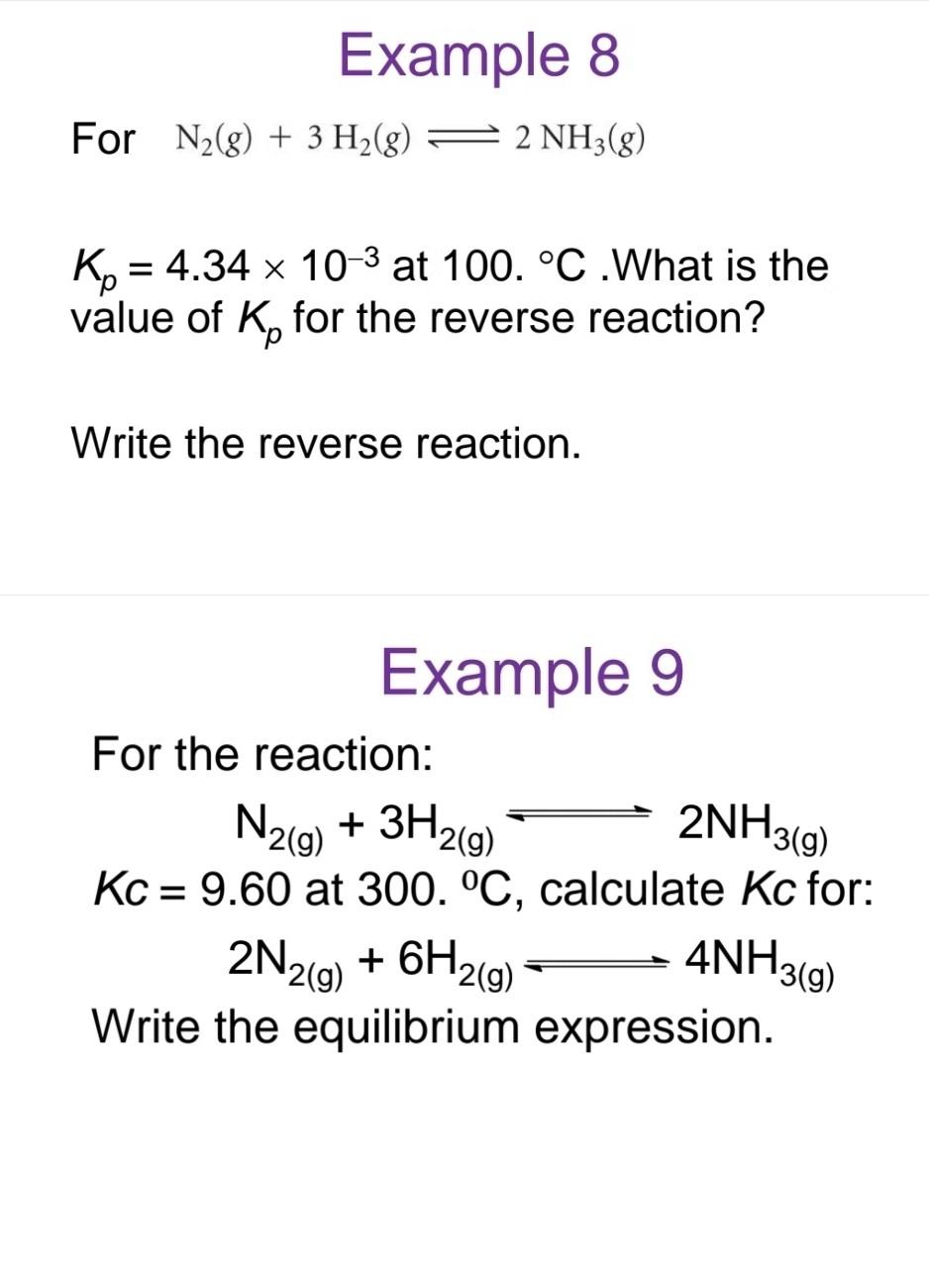
Example 8 For N2(g)+3H2(g)2NH3(g) Kp=4.34103 at 100.C. What is the value of Kp for the reverse reaction? Write the reverse reaction. Example 9 For the reaction: N2(g)+3H2(g)2NH3(g)Kc=9.60at300C,calculateKcfor:2N2(g)+6H2(g)4NH3(g) Write the equilibrium expression. An aqueous solution of acetic acid is found to have the following equilibrium concentrations at 25C : [CH3COOH]=1.65102M;[H+]=5.44104M; and [CH3COO]=5.44104M. Calculate the equilibrium constant Kc for the ionization of acetic acid at 25.0C. The reaction is CH3COOH(aq)H+(aq)+CH3COO(aq) Which side of the equilibrium is favoured? Example 6 For the reaction: H2(g)+I2(g)2HI(g) At 30C the equilibrium concentrations are - [I2]=0.00400M - [H2]=0.00400M - [HI]=0.0312M Find Kc and Kp Example 7 For the reaction: N2(g)+O2(g)2NO(g) At 25.0C,Kc=1.01030. Calculate the equilibrium constant for the reverse reaction: 2NO(g)N2(g)+O2(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


