Question: For the neutralization reaction below in which a strong base is reacted with a strong acid LOH+HClH2O+Na++Cl how many grams of lithium hydroxide are needed
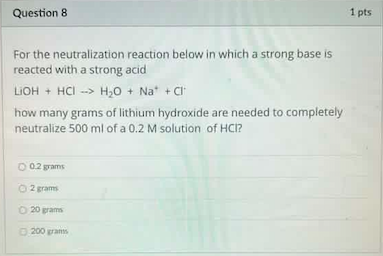
For the neutralization reaction below in which a strong base is reacted with a strong acid LOH+HClH2O+Na++Cl how many grams of lithium hydroxide are needed to completely neutralize 500ml of a 0.2M solution of HCl ? 0.2 grams 2 ram 20 grams 200 erans
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


