Question: From this data: 1. Find the theoretical yield for each concentration show only one calculation. 2. Find the actual and experimental yield for each concentration.
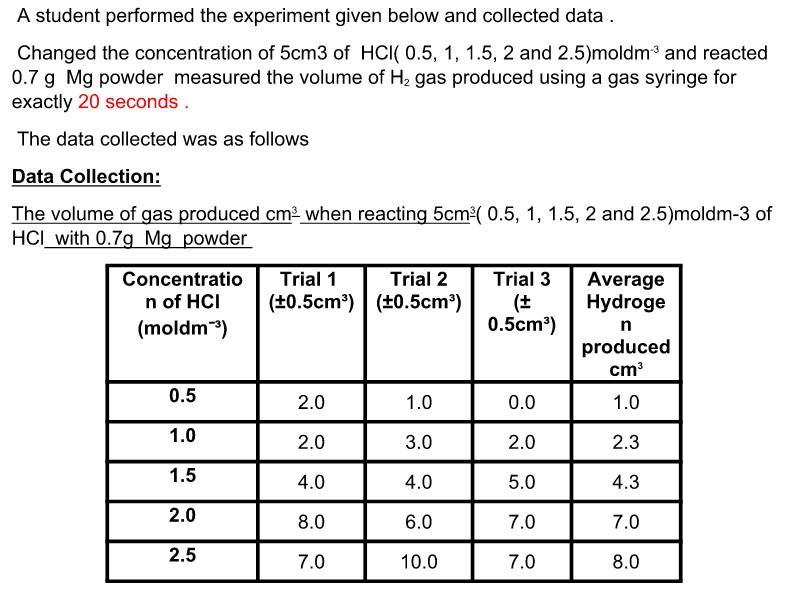 From this data:
From this data:
1. Find the theoretical yield for each concentration show only one calculation. 2. Find the actual and experimental yield for each concentration. Use 24dm3 of hydrogen gas as the experiment was conducted at RTP. 3. Find the rate of the reaction (look for the formula for the rate of the reaction and use correct units).
A student performed the experiment given below and collected data . Changed the concentration of 5cm3 of HCI 0.5, 1, 1.5, 2 and 2.5)moldm' and reacted 0.7 g Mg powder measured the volume of Hz gas produced using a gas syringe for exactly 20 seconds The data collected was as follows Data Collection: The volume of gas produced cm when reacting 5cm (0.5, 1, 1.5, 2 and 2.5)moldm-3 of HCL with 0.75 Mg powder Concentratio n of HCI (moldm) Trial 1 Trial 2 (+0.5cm) (+0.5cm) Trial 3 ( 0.5cm) Average Hydroge n produced cm 1.0 0.5 2.0 1.0 0.0 1.0 2.0 3.0 2.0 2.3 1.5 4.0 4.0 5.0 4.3 2.0 8.0 6.0 7.0 7.0 2.5 7.0 10.0 7.0 8.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


