Question: Given the binary equilibrium diagram, for the given conditions: P = 1 atm, T = 200 o C and X B = 50at%, what are
- Given the binary equilibrium diagram, for the given conditions: P = 1 atm, T = 200 oC and
XB = 50at%, what are the right phases and phase percentages? (Choose 1 option below, and show your calculation 2P)
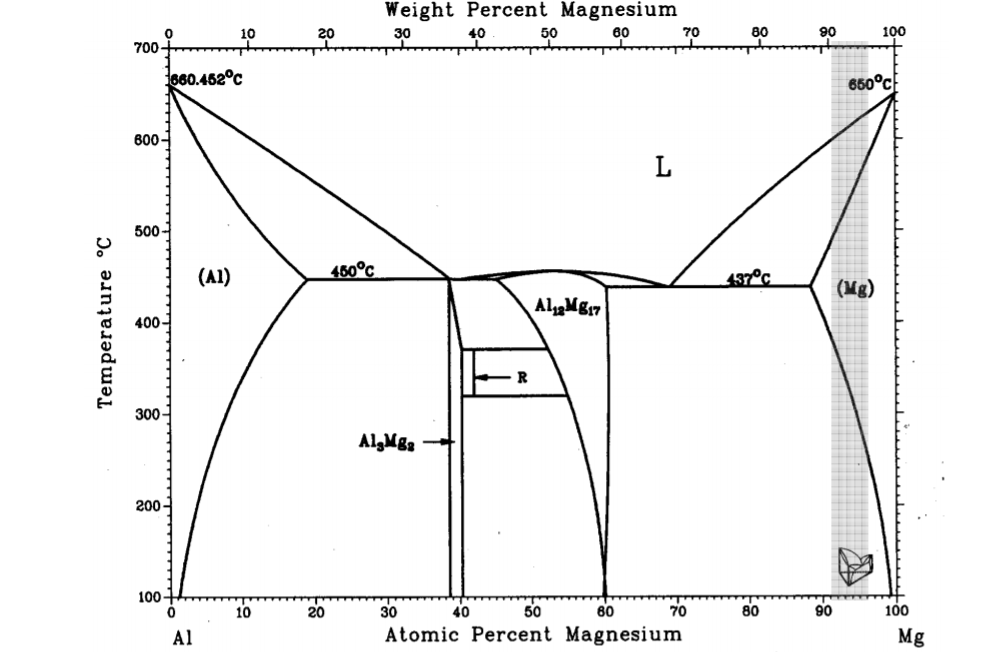
a) 10 Al3Mg2 - 90Al12Mg17 b) 50 Al3Mg2 - 50Al12Mg17 c)20 Al3Mg2 - 70Al12Mg17 d) 47 Al3Mg2 - 53Al12Mg17
e) 70 Al3Mg2 - 30Al12Mg17
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


