Question: Having tremendous trouble with this problem. Possibly with the significant figures. A 4.00(0.01)mL Class A transfer pipet is used to transfer 4.00mL of a 0.263(0.004)MCu2+
Having tremendous trouble with this problem. Possibly with the significant figures. 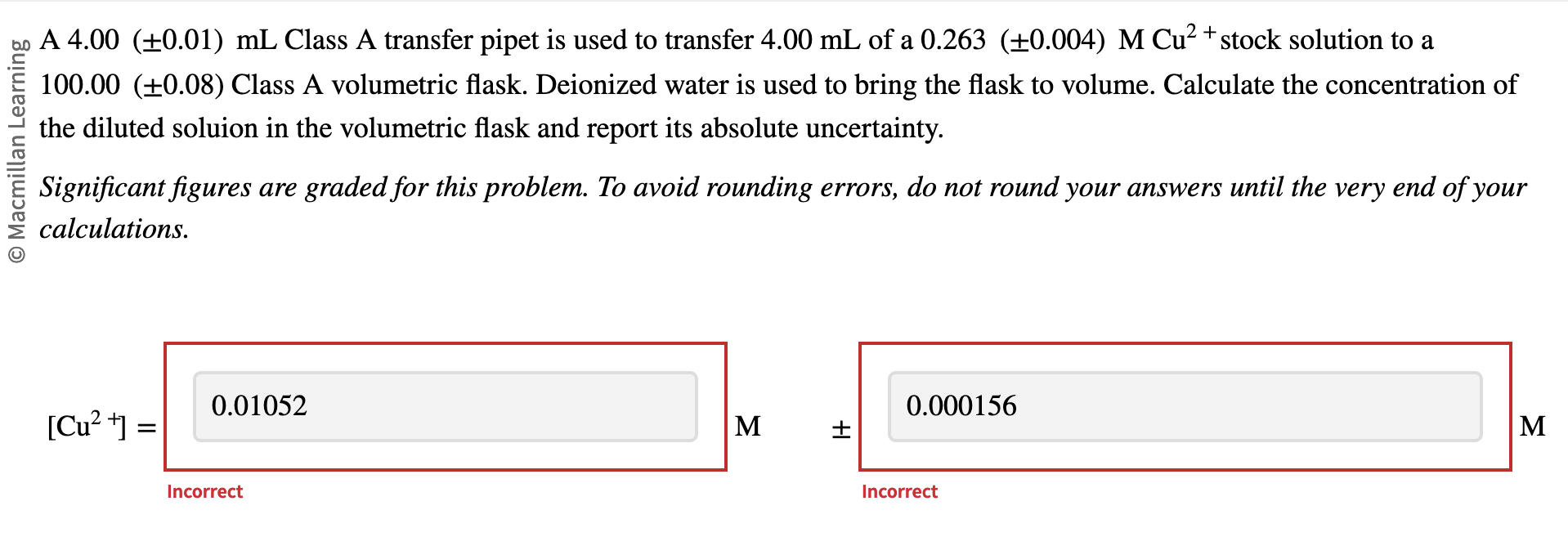
A 4.00(0.01)mL Class A transfer pipet is used to transfer 4.00mL of a 0.263(0.004)MCu2+ stock solution to a 100.00 ( 0.08) Class A volumetric flask. Deionized water is used to bring the flask to volume. Calculate the concentration of the diluted soluion in the volumetric flask and report its absolute uncertainty. Significant figures are graded for this problem. To avoid rounding errors, do not round your answers until the very end of your calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


