Question: Here we will not concern ourselves with Am as it really is just a degeneracy factor. AJ = 0 is allowed (Q branch) since the

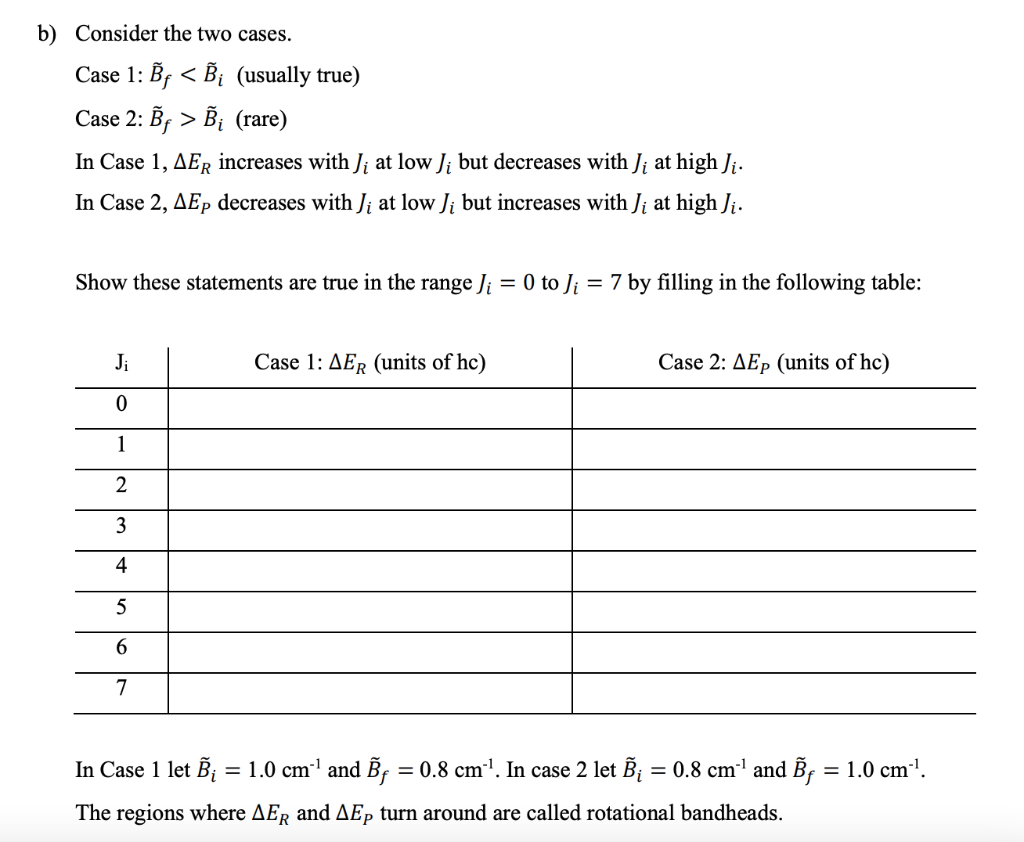
Here we will not concern ourselves with Am as it really is just a degeneracy factor. AJ = 0 is allowed (Q branch) since the electronic transition can take care of angular momentum issues. We will let Ji = rotational quantum number of the initial (i.e. ground) state Js = rotational quantum number of the final (i.e. excited) state There are three possibilities according to the selection rules: Jp = Ji - 1 P branch Js = Ji Q branch Jp = Ji +1 R branch Given: 2 Ei = = i;]i[i+1) = hcBJili +1) Es = 27,!; (5+1) = heBJsO+1) 2 = a) Show the following relationships are correct: P branch Of = Ji - 1) AEp = hc [(B: B;)J? (B; B;) Ji] Q branch (s = Ji) AEQ = hc Ji (i+1)(B, B;) R branch (s = Ji + 1) = AER = hc [(B: B;) Je? + BF (3J+ + 2) B; Ji] b) Consider the two cases. Case 1: B; B; (rare) In Case 1, AER increases with Ji at low Ji but decreases with Ji at high Ji. In Case 2, AEp decreases with Ji at low Ji but increases with Ji at high Ji. Show these statements are true in the range Ji = 0 to Ji = 7 by filling in the following table: Ji Case 1: AER (units of hc) Case 2: AEP (units of hc) 0 1 2 3 4 5 6 7 = In Case 1 let Xi = 1.0 cm' and BF = 0.8 cm"!. In case 2 let Xi = 0.8 cm- and By : = 1.0 cm? The regions where AER and AEp turn around are called rotational bandheads
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


