Question: Identify limiting reactants (maximum product method) Consider the reaction of tin with potassium hydroxide and water. Sn (s) + 2KOH (aq) + 4H20 (1) -K2Sn(OH).
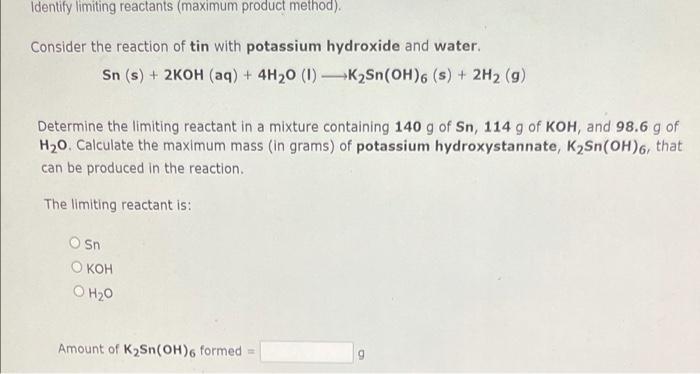
Identify limiting reactants (maximum product method) Consider the reaction of tin with potassium hydroxide and water. Sn (s) + 2KOH (aq) + 4H20 (1) -K2Sn(OH). (s) + 2H2 (9) Determine the limiting reactant in a mixture containing 140 g of Sn, 114 g of KOH, and 98.6 g of H20. Calculate the maximum mass (in grams) of potassium hydroxystannate, K Sn(OH)6, that can be produced in the reaction. The limiting reactant is: Osn O KOH O H20 Amount of K Sn(OH) formed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


