Question: In a previous example we found that the joule thompson coefficient for a gas entering a throttling valve: How do I show that if the
In a previous example we found that the joule thompson coefficient for a gas entering a throttling valve:
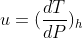
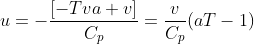
How do I show that if the gas entering the throttling valve is ideal, = 0, which means that the temperature of the exiting gas will be the same as the temperature of the entering gas?
Also:
Reduce the following partial derivatives to functions of CP, and kT .
(s/ P)v
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


