Question: Java coding 22 0 The approximate nuclear binding energy (Ex) of an atomic nucleus with atomic number Z and mass number A is calculated using
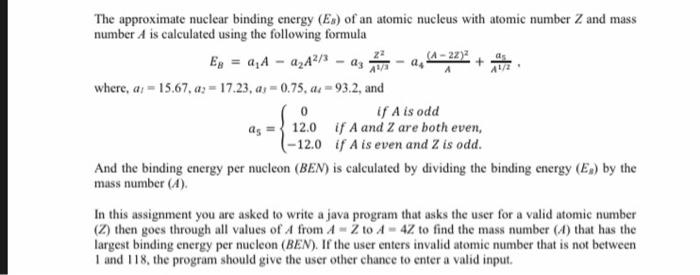
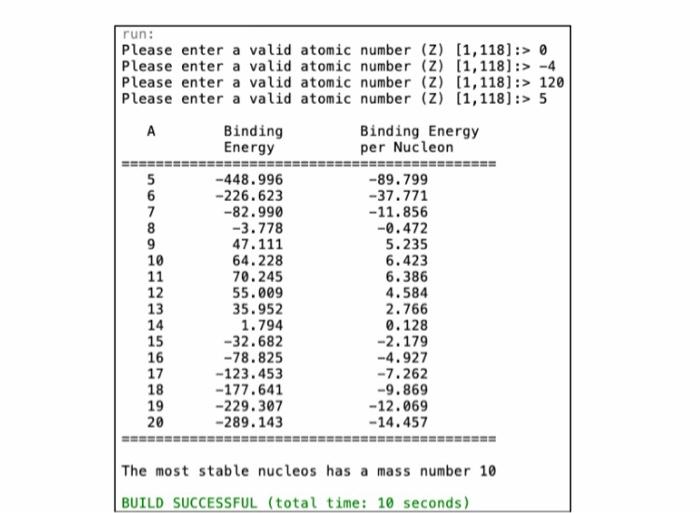
22 0 The approximate nuclear binding energy (Ex) of an atomic nucleus with atomic number Z and mass number A is calculated using the following formula (1-22) Eg = 2,4 - A2A2/3 - az where, a; - 15.67, az = 17.23, as = 0.75, ac = 93.2, and If A is odd 12.0 if A and 2 are both even, (-12.0 if A is even and Z is odd. And the binding energy per nucleon (BEN) is calculated by dividing the binding energy (E) by the mass number (A). In this assignment you are asked to write a java program that asks the user for a valid atomic number (2) then goes through all values of A from A - Z to A - 4Z to find the mass number (4) that has the largest binding energy per nucleon (BEN). If the user enters invalid atomic number that is not between 1 and 118, the program should give the user other chance to enter a valid input. run: Please enter a valid atomic number (Z) (1,118):> 0 Please enter a valid atomic number (Z) (1,118):> -4 Please enter a valid atomic number (z) (1,118) :> 120 Please enter a valid atomic number (2) (1,118) :> 5 . Binding Binding Energy Energy per Nucleon 5 -448.996 -89.799 6 -226.623 -37.771 7 -82.990 -11.856 8 -3.778 -0.472 9 47.111 5.235 10 64.228 6.423 11 70.245 6.386 12 55.009 4.584 13 35.952 2.766 14 1.794 0.128 15 -32.682 -2.179 - 78.825 -4.927 17 -123.453 -7.262 -177.641 -9.869 -229.307 -12.069 -289.143 -14.457 16 18 19 20 The most stable nucleos has a mass number 10 BUILD SUCCESSFUL (total time: 10 seconds)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


