Question: Use the followingexperimental titration data to calculatethe concentration of the acid being analysed. Observations: The initial solution of acetic acid (HC2H3O2) is clear and
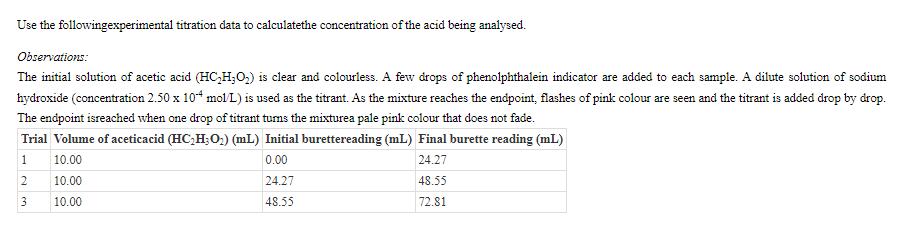
Use the followingexperimental titration data to calculatethe concentration of the acid being analysed. Observations: The initial solution of acetic acid (HC2H3O2) is clear and colourless. A few drops of phenolphthalein indicator are added to each sample. A dilute solution of sodium hydroxide (concentration 2.50 x 10 mol/L) is used as the titrant. As the mixture reaches the endpoint, flashes of pink colour are seen and the titrant is added drop by drop. The endpoint isreached when one drop of titrant turns the mixturea pale pink colour that does not fade. Trial Volume of aceticacid (HC2H3O2) (mL) Initial burettereading (mL) Final burette reading (mL) 1 10.00 2 10.00 3 10.00 0.00 24.27 48.55 24.27 48.55 72.81
Step by Step Solution
3.33 Rating (144 Votes )
There are 3 Steps involved in it
Solutions Let us rewrite the table at the very beginning Titration of Ace... View full answer

Get step-by-step solutions from verified subject matter experts