Question: Lithium borohydride (LiBH4) is a useful reducing agent, as it is more selective than LiAlH4 but less selective than NaBH4. Specifically, LiBH4 will reduce esters,
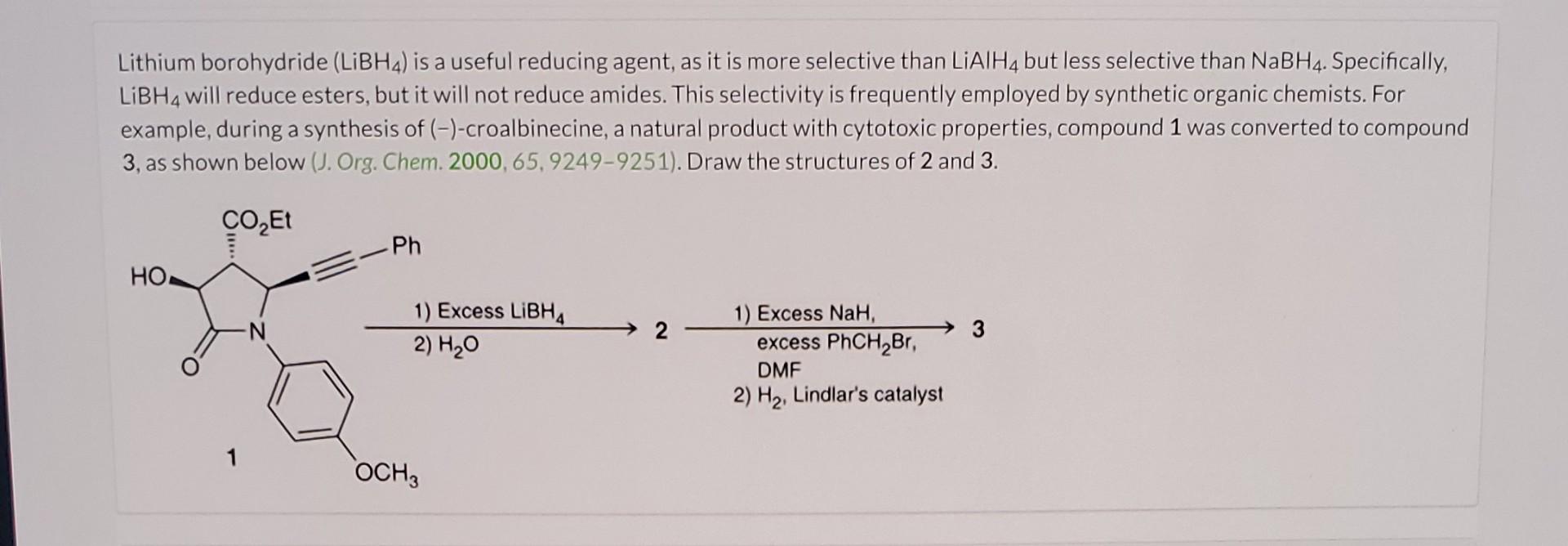
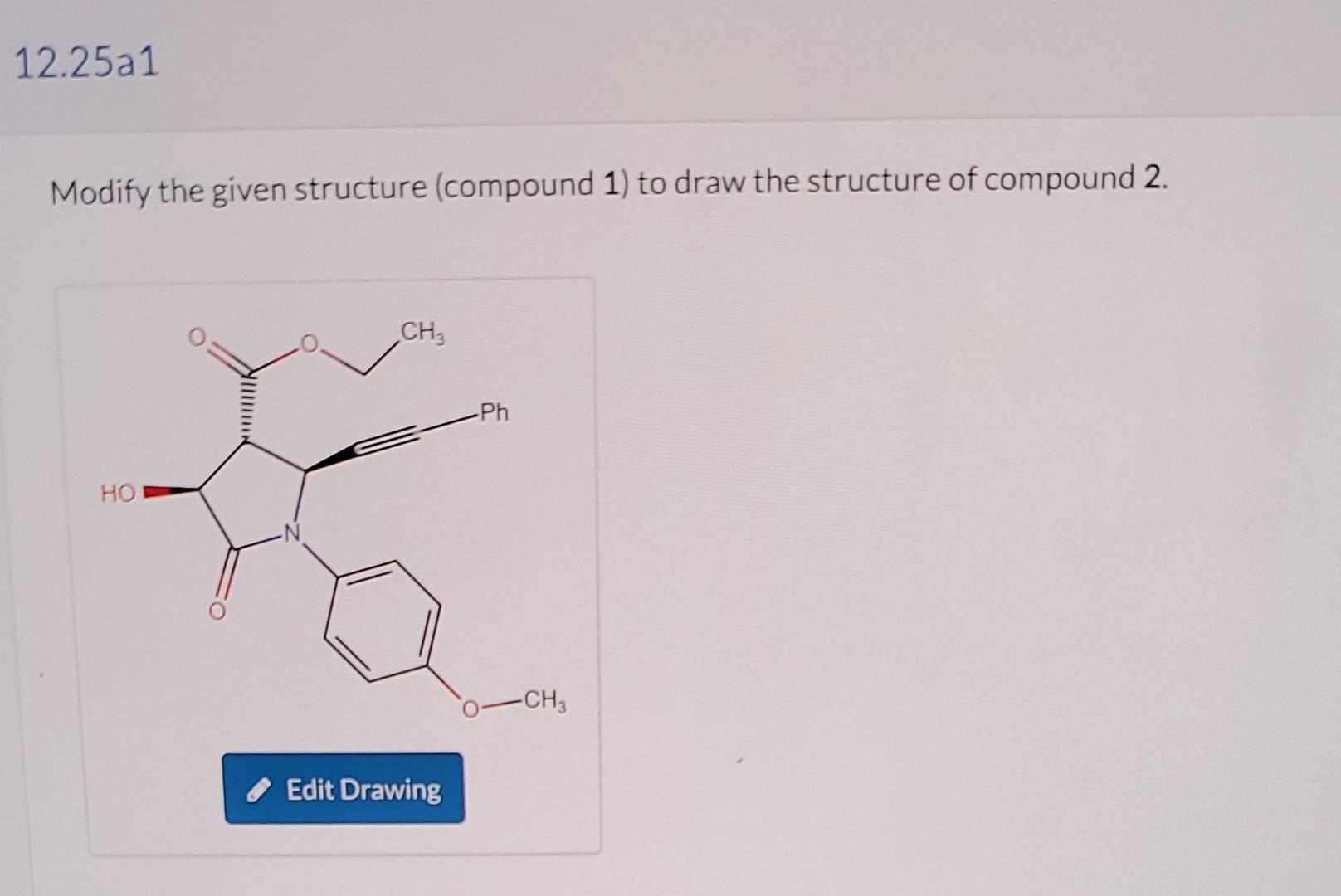
Lithium borohydride (LiBH4) is a useful reducing agent, as it is more selective than LiAlH4 but less selective than NaBH4. Specifically, LiBH4 will reduce esters, but it will not reduce amides. This selectivity is frequently employed by synthetic organic chemists. For example, during a synthesis of (-)-croalbinecine, a natural product with cytotoxic properties, compound 1 was converted to compound 3, as shown below (J. Org. Chem. 2000,65, 9249-9251). Draw the structures of 2 and 3. 1) Excess LiBH422)H2OexcessPhCH2Br11)ExcessNaH13 DMF 2) H2, Lindlar's catalyst Modify the given structure (compound 1) to draw the structure of compound 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


