Question: mass transfer a) The binary diffusion coefficient of a) CO2 in N2b) CO in O. c) CO2 in Hz are to be determined at the
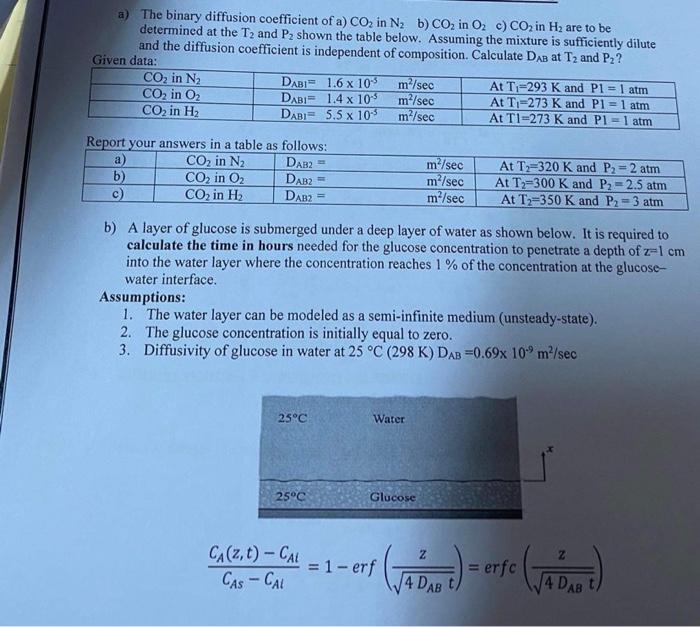
a) The binary diffusion coefficient of a) CO2 in N2b) CO in O. c) CO2 in Hz are to be determined at the T, and P2 shown the table below. Assuming the mixture is sufficiently dilute and the diffusion coefficient is independent of composition. Calculate DAB at T2 and Pz? Given data: CO2 in N2 DAB1.6 x 10 m/sec At T;=293 K and P1 = 1 atm CO, in O2 DABI 1.4 x 105 m/sec At T-273 K and P1 = 1 atm CO2 in H DABI= 5.5 x 10 m/sec At TI=273 K and Pl=1 atm Report your answers in a table as follows: a) CO2 in N2 DAB2 b) CO2 in O2 DAB2= c) CO2 in H DAB? m/sec m/sec m/sec At T=320 K and P2 = 2 atm At T2=300 K and P2 -2.5 atm At T2=350 K and P2 = 3 atm b) A layer of glucose is submerged under a deep layer of water as shown below. It is required to calculate the time in hours needed for the glucose concentration to penetrate a depth of z=1 cm into the water layer where the concentration reaches 1 % of the concentration at the glucose- water interface. Assumptions: 1. The water layer can be modeled as a semi-infinite medium (unsteady-state). 2. The glucose concentration is initially equal to zero. 3. Diffusivity of glucose in water at 25 C (298 K) DAB =0.69x 10.9 m/sec 25C Water 25C Glucose Z CA(2, t) CAL = 1 - erf CASCAL Gab. - erre (vb = erfc 14 DAB t 4 DAB
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


