Question: Need help with those three question. Structure B is the minor resonance contributor because :0:0 : A B O it has the most charge separation
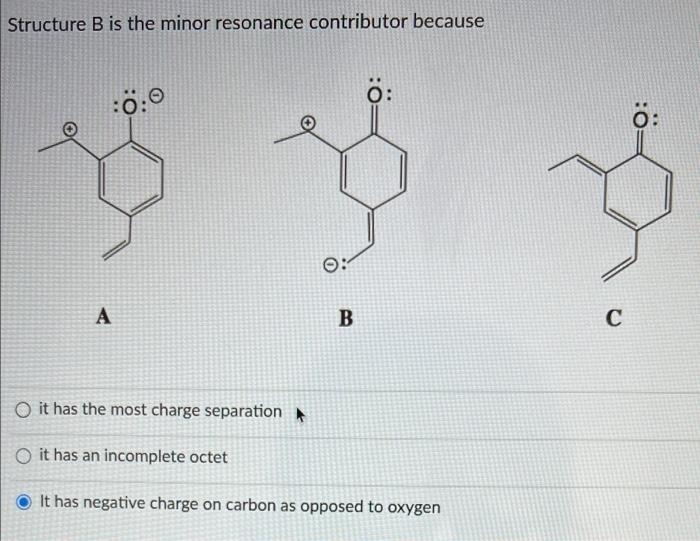
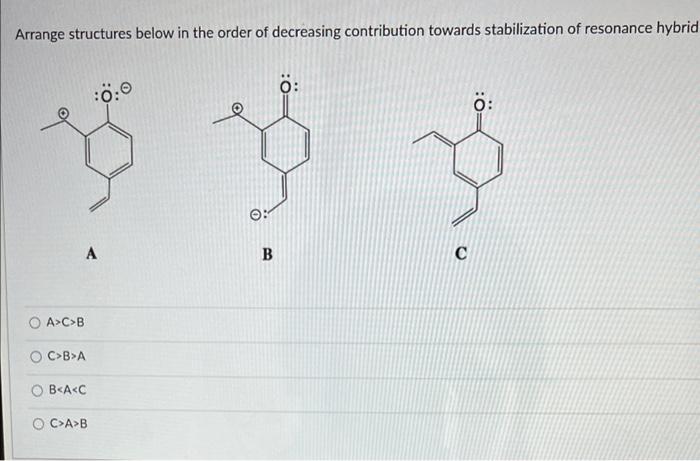
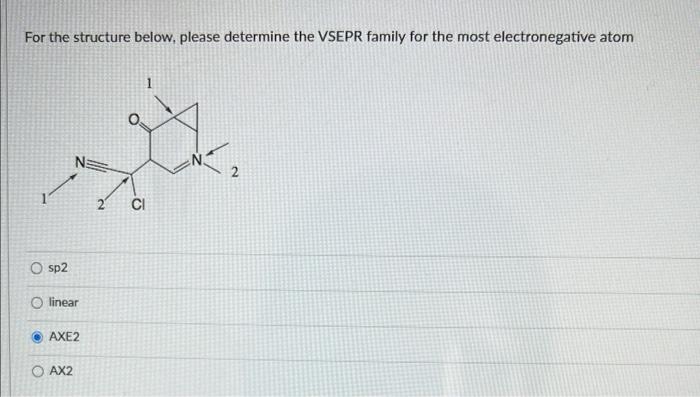
Structure B is the minor resonance contributor because :0:0 : A B O it has the most charge separation it has an incomplete octet It has negative charge on carbon as opposed to oxygen Arrange structures below in the order of decreasing contribution towards stabilization of resonance hybrid : YO 0: A B O AC>B OC>B>A BA>B For the structure below, please determine the VSEPR family for the most electronegative atom NE CI O sp2 linear AXE2 O AX2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


