Question: on 2 The chart below shows the pressure - composition diagram of the binary mixture of methane and propane at 15 C. (15 x 2
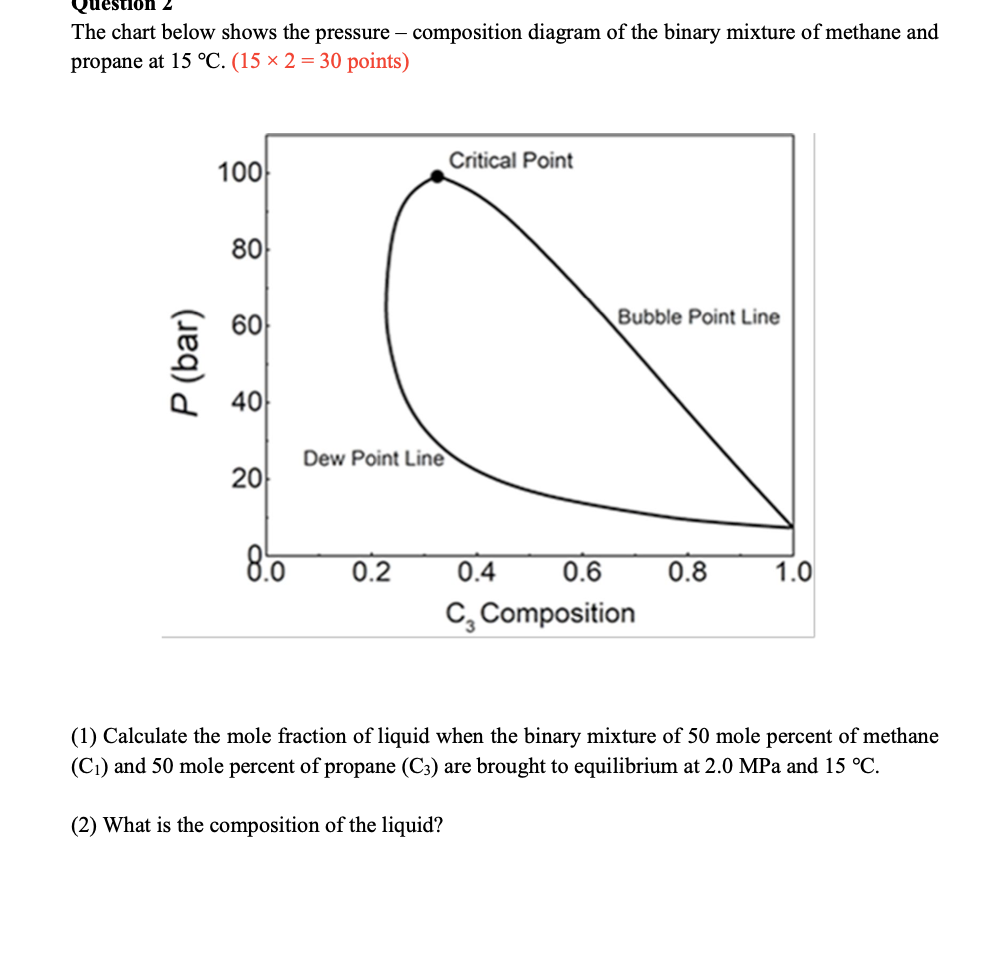
on 2 The chart below shows the pressure - composition diagram of the binary mixture of methane and propane at 15 C. (15 x 2 = 30 points) 100 Critical Point 80 60 Bubble Point Line P (bar) 40 Dew Point Line 20 8.6 0.2 0.8 1.0 0.4 0.6 C, Composition (1) Calculate the mole fraction of liquid when the binary mixture of 50 mole percent of methane (C1) and 50 mole percent of propane (C3) are brought to equilibrium at 2.0 MPa and 15 C. (2) What is the composition of the liquid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


