Question: our reactions are given below. For the first, pre the products, write the balanced chemical equation, and classify it as a precipitation, acidase, gas-forming, or
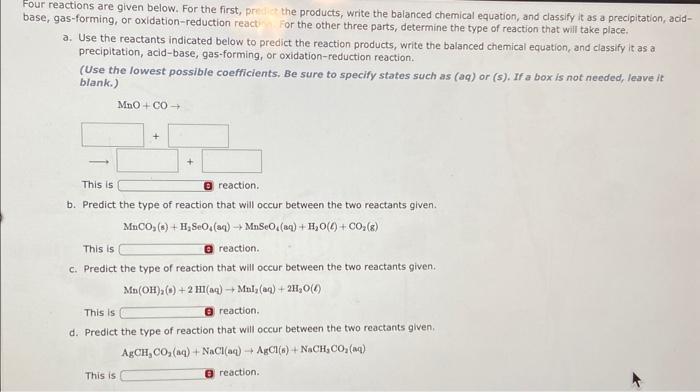
our reactions are given below. For the first, pre the products, write the balanced chemical equation, and classify it as a precipitation, acidase, gas-forming, or oxidation-reduction react For the other three parts, determine the type of reaction that will take place. a. Use the reactants indicated below to predict the reaction products, write the balanced chemical equation, and classify it as a precipitation, acid-base, gas-forming, or oxidation-reduction reaction. (Use the lowest possible coefficlents, Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) b. Predict the type of reaction that will occur between the two reactants given. MnCO2(s)+H2SeO4(aq)MnSeO4(sq)+H2O()+CO2(g) This is reaction. c. Predict the type of reaction that will occur between the two reactants given. Mn(OH)2(s)+2HI(aq)Mnl2(aq)+2H2O() This is ireaction. d. Predict the type of reaction that will occur between the two reactants given. AgCH3CO2(aq)+NaCl(aq)+AgCl(6)+NaCH3CO2(aq) This is reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


